Acetyl CoA Detected Fluorescent Probes
RH-NH2 is a fluorescent probe with a tetramethylrhodamine skeleton known to localize to mitochondria. The fluorescence intensity of this probe increases when the amino group is acetylated, making it possible to detect intracellular acetyl CoA.
About Acetyl CoA
Acetyl CoA is an important intermediate that plays a central role in energy metabolism. When carbohydrates, amino acids, and lipids are broken down for energy, most of them are oxidized to acetyl CoA in the TCA cycle. At the same time, acetyl CoA is the donor of the acetyl group in the acetylation reaction of histone. Histone is basic proteins found in the nucleus, and histone acetylation generally works to promote gene expression. This is because the addition of acetyl groups to histone tails by histone acetyltransferases reduces the positive charge of histone and the electrical interaction between histone and DNA, which loosens the aggregated nucleosome structure and allows RNA polymerase to bind to promoter regions (Figure 1). Conversely, when expression is suppressed, acetyl groups are removed by histone deacetylases, resulting in stronger binding of histones to DNA and suppression of gene expression. In cancer, it has been reported that the expression of cancer suppressor genes is negatively regulated by deacetylation of histone, and histone deacetylases are attracting attention as a target of anticancer drugs. Hence, acetyl CoA has been shown to play an important role in metabolic regulation and gene transcription regulation, and there is a need for an easy method to assay acetyl CoA in cells.
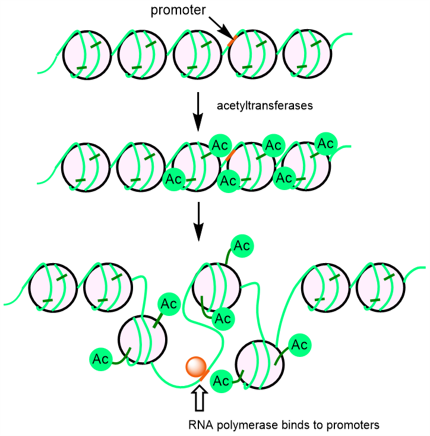
Figure 1. Alteration of chromatin structure by histone acetylation
About The Fluorescent Probe RH-NH2
To detect intracellular acetyl CoA as a fluorescent signal, a molecule whose conformational change is induced by acetyl CoA and increases the fluorescence intensity is required. Dr. Yamatsugu and co-worker wondered if they could transfer acetyl groups from acetyl CoA to fluorescent probes in cells by using a reaction accelerator that transfers acetyl groups from acetyl CoA together with fluorescent probes, thereby increasing the fluorescence intensity. Therefore, they designed a fluorescent probe, RH-NH2, whose fluorescence intensity increases when its amino group undergoes acetylation, which triggers the photoinduced electron transfer (PET) mechanism, using N,N,N',N'-tetramethylrhodamine, which has been reported to localize in mitochondria where acetyl CoA is produced, as the matrix of the fluorescent probe (Figure 2). RH-NH2 has a maximum absorption wavelength at 546 nm (ε = 71400 M-1 cm-1) and a maximum fluorescence wavelength at 570 nm, while its acetylated form, RH-NHAc, has a maximum absorption wavelength at 553 nm (ε = 35500 M-1 cm-1) and a maximum fluorescence wavelength at 572 nm. The fluorescence quantum yield of RH-NH2 in PBS buffer (1% DMSO) is 4.9 x 10-4, whereas that of its acetylated form, RH-NHAc, is 0.12, showing approximately 50 times higher fluorescence intensity at 520 nm excitation (Figure 3a)). Thus, RH-NH2 is a turn-on type fluorescent probe whose fluorescence intensity increases when its amino group undergoes acetylation.
They investigated reaction accelerators that promote the transfer of acetyl groups from acetyl CoA to RH-NH2 and found that tributylphosphine (PBu3) is the most efficient accelerator for the transfer of acyl groups to RH-NH2. Figure 3b shows the time courses of the fluorescence intensity of RH-NH2 when Thioester 1 (Figure 2), which is the same thioester as acetyl CoA, was used as an acetylating agent and PBu3 as a reaction accelerator. The fluorescence intensity increased rapidly when PBu3, a reaction accelerator, was added 1 minute after the start of the measurement in the presence of RH-NH2 and Thioester 1. The degree of increase was 2- to 3-fold, which is lower than the 50-fold increase in fluorescence intensity when RH-NH2 is fully acetylated (Figure 3a), but still indicates that acetyl CoA of the order of mM can be detected.
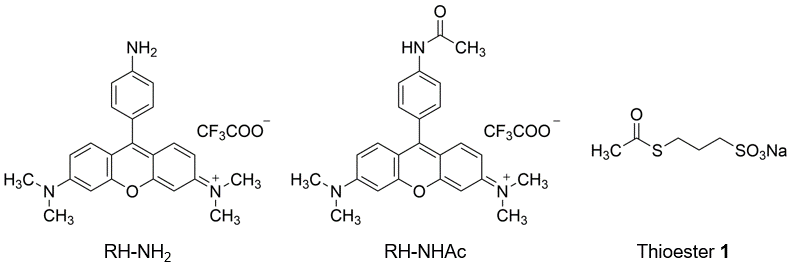
Figure 2. Structure of Fluorescent Probe RH-NH2
RH-NH2 (left), the acetylated product RH-NHAc (center), acetyl CoA equivalents thioester 1 (right)
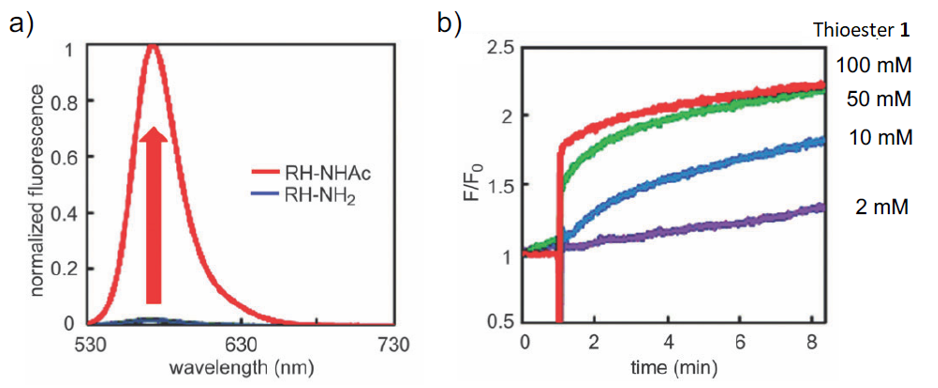
Figure 3. Fluorescent Characteristic of RH-NH2
a) Fluorescence spacta of RH-NH2 and RH-NHAc (excited at 520 nm)
b) Time courses of the fluorescence enhancement in PBu3-promoted acetylation of RH-NH2 in PBS (pH 7.4, 1% DMSO) at 25 degree.
When HeLa S3 cells are treated with RH-NH2 (2 µM), they localize to the mitochondria and emit weak fluorescence, as expected. Although no increase in fluorescence intensity was observed at this point, when PBu3 DMSO solution (5 mM) , an acetyltransfer accelerator, was added, a clear increase in fluorescence intensity was observed within 1 minute (Figure. 4). This is thought to be because PBu3 activates acetyl CoA to acetylate the RH-NH2 probe localized in the mitochondria, resulting in the increase in fluorescence intensity. It should be noted that the addition of PBu3 (5 mM) or RH-NH2 (10 µM) did not cause cytotoxicity and mitochondrial membrane potential changes.
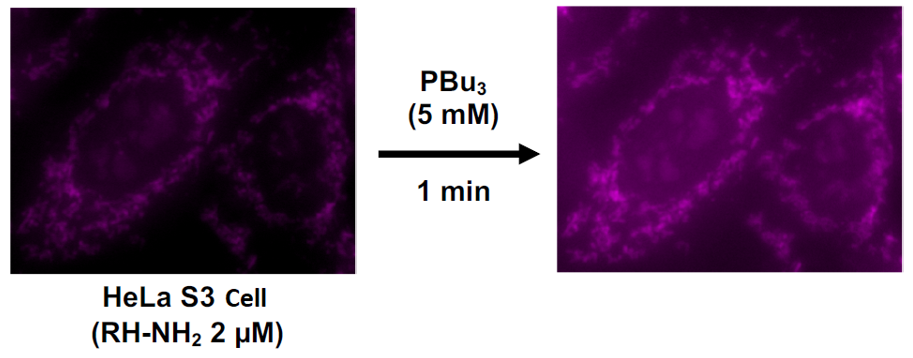
Figure 4. Fluorescence detection of intracellular acetyl CoA by RH-NH2
Reference
Komatsu, H. et al. : Chem. Commun., 49 (28), 2876 (2013).
Product List
- Open All
- Close All
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



