JQ Vial Kit

JQ Vial Kit is an "all made in Japan" vial kit comprising a glass vial and a septum cap, both made in Japan. The vial is manufactured by a unique method with an optimized processing temperature in the high-temperature molding process, enabling low adsorption and low alkaline elution without chemical treatment with silane, etc. The septum cap is also of high quality, minimizing elution of impurities.

In chromatographic analysis such as GC and LC, there are risks of unexpected analytical defects such as sample adsorption, decomposition, and appearance of ghost peaks, depending on the quality of the sample containers, the vial and septum cap. As the recent developments in analytical equipment and technology bring a need for ever more sophisticated analysis, it is critical to focus on the quality of the sample containers as well.
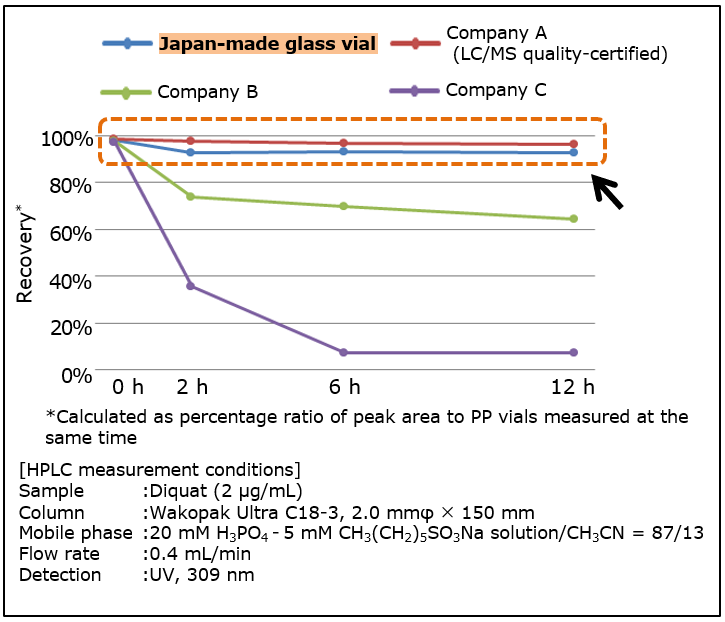
Comparison of Sample Adsorption to Vials
Low adsorption equivalent to LC/MS quality certified product of competitor.
Sample adsorption to vials was tested to compare our Japan-made glass vial with vial products from other companies. Diquat, used as herbicide and known to be highly adsorptive to glass, was used as sample. After sealing diquat solution into a vial, recovery up to 12 hours was measured by HPLC.
-
Diquat adsorption to Japan-made glass vial was hardly observed up to 12 hours, demonstrating as low adsorption as LC/MS certified high-quality glass vials from competitor. As sample adsorption to vials causes sample loss, use of low-adsorption vials is important to avoid problems in analysis.
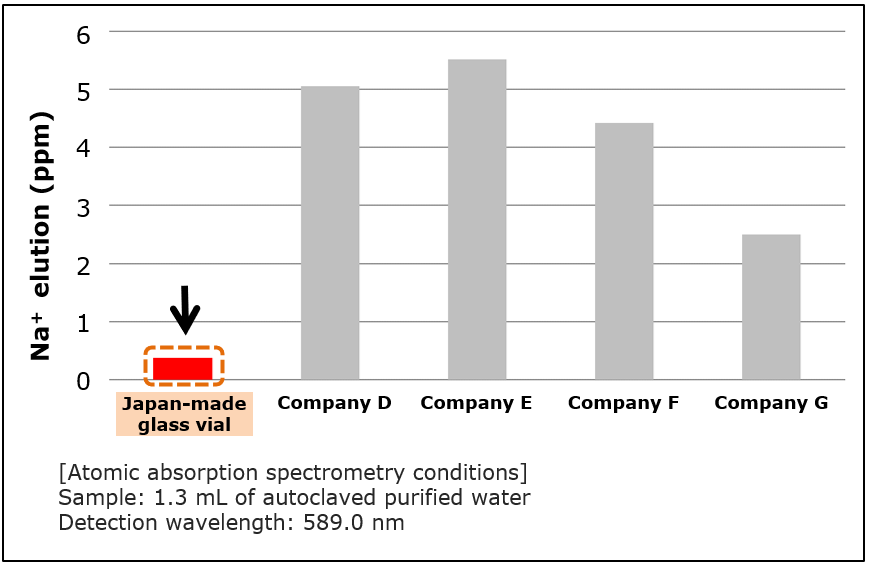
Comparison of Na+ Elution from Vials
Significantly low Na+ elution compared to competitor’s product.
Na+ elution from vials was tested to compare our Japan-made glass vial with vial products from other companies. After sealing purified water into vials, Na+, known to be an alkaline component eluted from glass, was measured by atomic absorption spectrometry.
-
-
Japan-made glass vial was shown to be associated with low Na+ elution. As elution of alkaline components from vials causes sample decomposition, etc., it is critical to pay attention not only to sample adsorption to vials, but also elution from vials.
Comparison of Impurities Elution from Septum Caps
Almost no elution from the septum cap.
Impurities elution from septum caps was tested to compare our Japan-made septum cap with septum cap products from other companies. Leachables in each vial were measured by GC/MS 10 times consecutively.
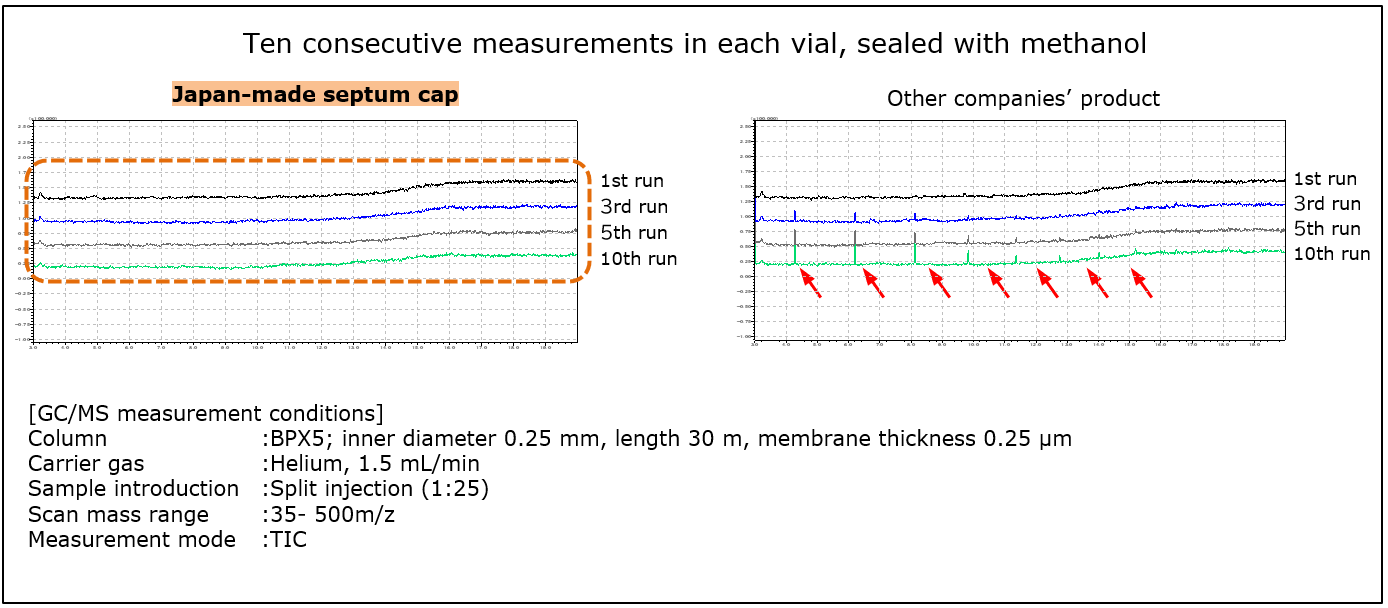
| In consecutive measurements, leachables observed with other companies' products were hardly detected with Japan-made septum cap. Leachables from a cap or septum can have unexpected effects on test results, such as appearance of ghost peaks. Thus, it is necessary in analytical testing to pay attention to the quality not only of vials, but also of septum caps. |
Product Information
| Manufacturers of compatible products | Shimadzu, Hitachi, Waters, Agilent, etc. |
|---|---|
| Glass material | Borosilicate glass *low adsorption, low Na+ elution |
| Septum material | Fluororesin/silicone *low impurities elution |
| Specification of screw-top | 9-425 |
Product List
- Open All
- Close All
JQ Vial Kit (Screw vial, 1.5ml Transparent+Septum Screw Cap, Fluoropolymer/Silicone)
JQ Vial Kit (Screw vial, 1.5ml Transparent+Septum Screw Cap, Fluoropolymer/Silicone, Pre-slit)
JQ Vial Kit (Screw vial, 1.5ml Transparent+Write-on Spot+Septum Screw Cap, Fluoropolymer/Silicone)
JQ Vial Kit (Screw vial, 1.5ml Transparent+Write-on Spot+Septum Screw Cap, Fluoropolymer/Silicone, Pre-slit)
JQ Vial Kit (Screw vial, 1.5ml Brown+Septum Screw Cap, Fluoropolymer/Silicone)
JQ Vial Kit (Screw vial, 1.5ml Brown+Septum Screw Cap, Fluoropolymer/Silicone, Pre-slit)
JQ Vial Kit (Screw vial, 1.5ml Brown, Write-on Spot+Septum Screw Cap, Fluoropolymer/Silicone)
JQ Vial Kit (Screw vial, 1.5ml Brown, Write-on Spot+Septum Screw Cap, Fluoropolymer/Silicone, Pre-slit)
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



