[Fushimi Pharmaceutical] Glycan Related Products
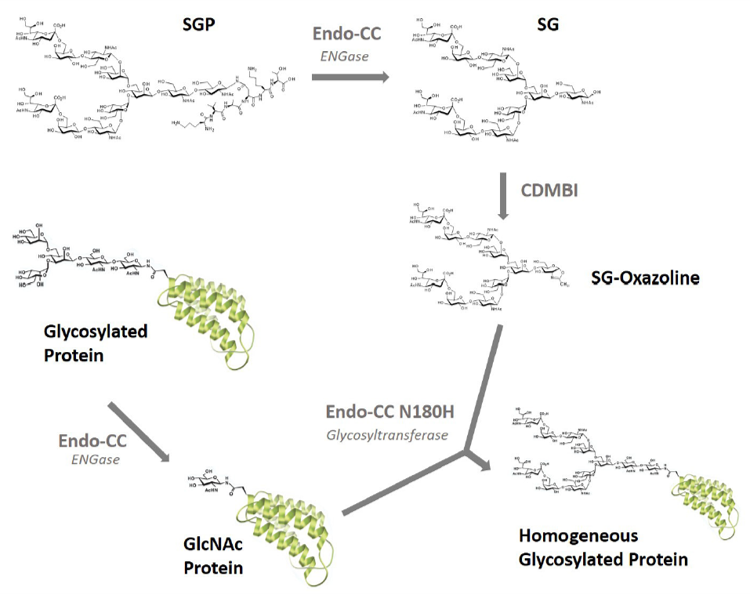
Glycans (sugar chain) contain of a large number of linked monosaccharides. Glycoproteins, composed of glycan and protein, are known to play a major role in biological systems and are involved in many biological processes. Glycans of natural origin are heterogeneous and complexed structures and therefore very challenging to analyze. Due to this attribute, the synthesis of homogeneous glycans was considered to be difficult.
Fushimi Pharmaceutical Co., Ltd. developed a technology to produce large amounts of homogeneous N-glycans from egg yolk with high purity, which is also useful for analyzing glycans with complex structures. In addition, Fushimi Pharmaceutical has a lineup of products for attaching the glycans to the glycosylation position of glycoproteins.
Transglycosylation Peptides/Proteins
Glycopeptide SGP
Features
- Raw material for bio-pharmaceuticals
- Analytical reagents to assay viruses
- Reagent for glycol research (raw material for glycolibrary)
▼ Sialylglycopeptide (SGP; α2,6-SGP)
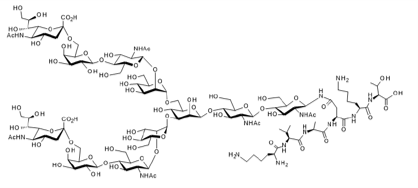
Chemical Formula: C112H189N15O70
Mol. Weight: 2,865.78
CAS No.: 189035-43-6
Purity: min. 95% (HPLC)
Product Number: 386-02374; 382-02371;388-02373
▼α2,3-Sialylglycopeptide (α2,3-SGP)
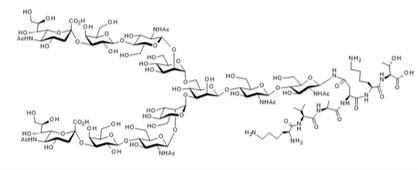
Chemical Formula: C112H189N15O70
Mol. Weight: 2,865.78
CAS No.: 426211-96-3
Purity: min. 95% (HPLC)
Product Number: 381-10151; 387-10153
▼Asialoglycopeptide (Asialo-SGP)
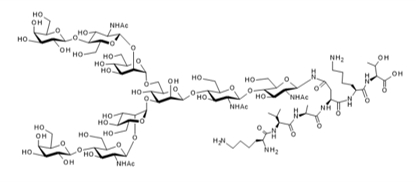
Chemical Formula: C90H155N13O54
Mol. Weight: 2,283.23
CAS No.: 361443-81-4
Purity: min. 95% (HPLC)
Product Number: 388-10161; 384-10163
▼ Agalactoglycopeptide (Agalacto-SGP)
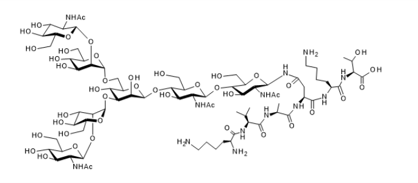
Chemical Formula: C79H139N13O43
Mol. Weight: 1959.01
CAS No.: 361443-82-5
Purity: min. 95% (HPLC)
Product Number: 381-19171; 387-19173
SGP Derivatives
Features
- for sugar chain homogenization and sugar chain replacement
▼ α2,6-Sialylglycan (α2,6-SG)
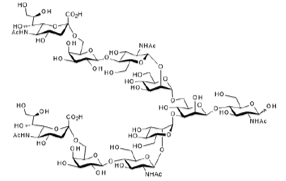
Chemical Formula: C76H125N5O57
Mol. Weight: 2,020.80
CAS No.: 58902-60-6
Purity: min. 95% (HPLC)
Product Number: 385-10176; 385-10171; 389-10174
▼ Asialoglycan (G2-glycan)
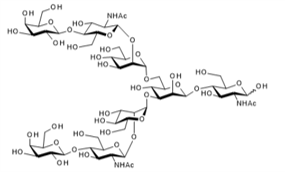
Chemical Formula: C54H91N3O41
Mol. Weight: 1,438.30
CAS No.: 52789-60-3
Purity: min. 95% (HPLC)
Product Number: 388-19181; 384-19183
▼ Agalactoglycan (G0-Glycan)
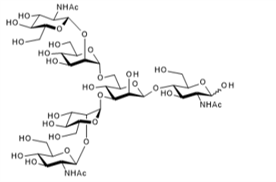
Chemical Formula: C42H71N3O31
Mol. Weight: 1,114.01
CAS No.: 61687-27-2
Purity: min. 95% (HPLC)
Product Number: 385-19191; 381-19193
▼ Oxazoline-Sialylglycan(Oxazoline-SG)
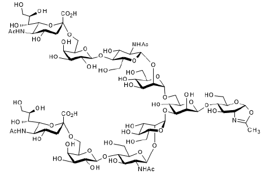
Chemical Formula: C76H123N5O56
Mol. Weight: 2,002.79
Purity: min. 95% (HPLC)
Product Number: 388-10183; 382-10181
▼ Asialoglycan-Oxazoline (G2-Oxazoline, Asialo-Oxazoline)
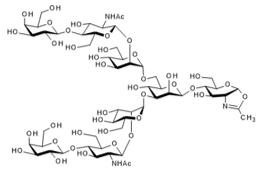
Chemical Formula: C54H89N3O40
Mol. Weight: 1,420.28
CAS No.: 1118943-57-9
Purity: min. 95% (HPLC)
Product Number: 387-19151; 383-19153
▼ Agalactoglycan-Oxazoline (G0-Oxazoline)
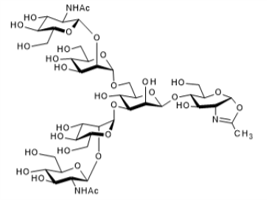
Chemical Formula: C42H69N3O30
Mol. Weight: 1,096.00
CAS No.: 1186583-29-8
Purity: min. 95% (HPLC)
Product Number: 384-19161; 380-19163
Oxazoline-forming agent
Features
- stable in water/ easy to handle
- promotes one-pot glycosyl-transfer reaction
- only small amount of reagent is required
- is used for the preparation of glycan donors
▼ CDMBI (2-Chloro-1,3-dimethyl-1H-benzimidazol-3-ium chloride)
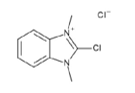
Chemical Formula: C9H10ClN2Cl
Mol. Weight: 217.10
CAS No.: 364774-78-7
Purity: min. 95% (HPLC)
Product Number: 380-02671, 386-02673, 388-02672
Enzymes
Features
- Endo-β-N-acetylglucosaminidase (ENGase)
- Endo-CC catalyzes hydrolysis of N-linked oligosaccharide
- Endo-CC N180H is Endo-CC mutant, and can transfer sugar chains for example SG-Oxazoline as donor to GlcNAc as acceptor
▼ Endo-CC
Package size: 300 mU
Source: Endo-CC recombinant from Coprinopsis cinerea is expressed in E.coli as a fusion to His tag.
▼ Endo-CC N180H
Package size: 300 mU
Source: Endo-CC N180H recombinant from Coprinopsis cinerea is expressed in E.coli as a fusion to His tag.
Product List
- Open All
- Close All
Glycopeptide SGP
SGP Derivatives
Reagent for Sugar Oxazolination
Deglycosylation/Glycosylation Enzymes
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



