Thiol Detection Probes
Thiol groups play a crucial role in determining protein structure, primarily through the formation of disulfide bonds (S-S bonds). However, because protein thiol groups exhibit high nucleophilicity, they are highly susceptible to oxidation by reactive oxygen species (ROS). Excessive oxidation of thiol groups caused by ROS-mediated oxidative stress leads to structural alterations and denaturation of proteins, resulting in adverse effects on biological systems.
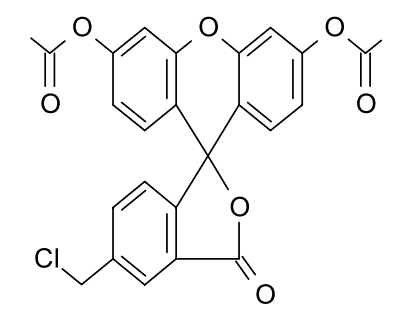
For imaging intracellular thiol groups, fluorescent probe-based methods have become the predominant approach. Commonly used fluorescent probes include 7-diethylamino-3-(4-maleimidophenyl)-4-methylcoumarin and CMFDA (5-chloromethylfluorescein diacetate).
Features
Thiol groups (–SH) are functional groups consisting of a sulfur atom bonded to hydrogen. Among the amino acids that constitute proteins, thiol groups are found in cysteine and play a crucial role in determining protein structure, primarily through the formation of disulfide bonds (S–S bonds). However, because protein thiol groups exhibit high nucleophilicity, they are highly susceptible to oxidation by reactive oxygen species (ROS). Excessive oxidation of thiol groups caused by ROS-mediated oxidative stress leads to structural alterations and denaturation of proteins, resulting in adverse effects on biological systems. On the other hand, oxidative modifications of thiol groups play essential roles in biological processes such as signal transduction and metabolism.
In cells, the tripeptide glutathione plays a key role in preventing excessive oxidation of thiol groups. The thiol group of reduced glutathione (GSH) is oxidized by reactive oxygen species, such as hydrogen peroxide, and converted to glutathione disulfide (GSSG; glutathione-S-S-glutathione). Because GSH is present at high intracellular concentrations, excessive oxidation of functionally important proteins is suppressed. In recent years, it has also become clear that, in addition to thiol groups, selenol groups (–SeH), which contain selenium in their structure, suppress oxidation caused by ROS . Therefore, selenol groups are also considered to play important roles in antioxidant activity, similar to thiol groups1).
For the quantification of thiol groups, methods using reagents such as 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB; Ellman’s reagent) have long been employed. In contrast, for imaging intracellular thiol groups, fluorescent probe–based methods have become the predominant approach. Commonly used fluorescent probes include 7-diethylamino-3-(4-maleimidophenyl)-4-methylcoumarin and CMFDA (5-chloromethylfluorescein diacetate).
ViVidFluor Cell Green CMFDA
ViVidFluor Cell Green CMFDA is a fluorescent dye that reacts with intracellular thiol groups. It readily permeates the plasma membrane of live cells and is hydrolyzed by intracellular esterases, becoming fluorescent. After binding to intracellular thiol groups, it is retained within the cell, enabling prolonged imaging.
Features
- Reacts with intracellular thiol groups
- Enables prolonged imaging
- Ex/Em=492 nm/516 nm
References
- Roman, M., Jitaru, P. and Barbante, C.: Metallomics., 6(1), 25(2014).
Selenium biochemistry and its role for human health
Product List
- Open All
- Close All
CMFDA
7-diethylamino-3-(4-maleimidophenyl)-4-methylcoumarin
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



