Kinetic Turbidimetric Assay (Onset time method)
This article was written by Dr. Masakazu Tsuchiya, FUJIFILM Wako Pure Chemical Corporation, for Vol. 59, No. 3 (October 1991) of Wako Junyaku Jiho.
The content of this article is from the time of publication. It is not the latest information due to new knowledge and changes in regulatory rules after original publication.
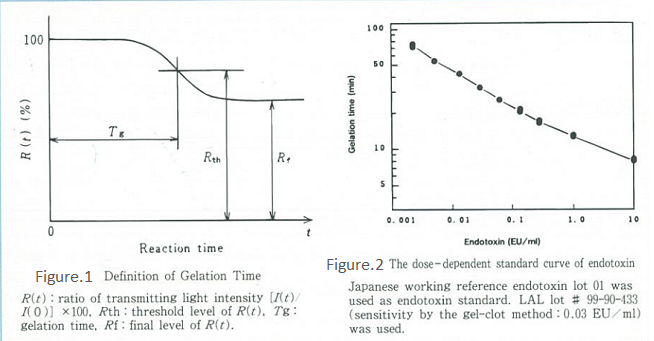
The kinetic turbidimetric assay (Onset time method) is listed as the kinetic-turbidimetric technique in FDA Guidelines1) and is now widely used as part of Limulus test. Our Toxinometer® is a specialized device for the kinetic turbidimetric assay. It observes the gelation process as a change in the intensity of light transmission through a sample. The time at which the ratio of transmitting light intensity changes to a certain fixed ratio is defined as the gelation time (Tg), and endotoxin concentration can be quantified based on this Tg value.
Figure 1 shows the schematic diagram of the principle, while Figure 2 shows an example of the calibration curve. Although this method requires a specialized device, it is easy to operate and very objective as the device automatically determines the gelation time. In addition to being very sensitive, quantitative, and economically efficient in terms of reagents, this method also has an extremely wide calibration range compared with other methods.
Previously, the concept of the kinetic turbidimetric assay has been reported by Jorgensen et al. 2, 3); however, it was not put into practical application because there was no specialized device on the market. Oishi et al.4, 5) were the first in the world to manufacture a parallel kinetic turbidimetric assay device that can measure transmitting light intensity of multiple samples in parallel. This device had the advantage of being able to cancel the differences in light source intensity of the optical system and sensor sensitivity by calculating the ratio of transmitting light intensity of each sample. After rigorous testing in external evaluations, the device was commercialized as the Toxinometer®.
Other kinetic turbidimetric assay devices include the LAL-5000 of Associates of Cape Cod, Inc. (USA). The basic principle of these devices is similar, but in the LAL-5000, 400µl of sample is added to 100µl of LAL, and determination through the gel-clot technique is thus not possible. These devices are also approved by the FDA.
The reported applications of the kinetic turbidimetric assay include application in quality control production processes6), measurement of endotoxin in water7), measurement of endotoxin in blood products8), detection of Gram-negative bacteria9), and measurement of endotoxin in blood10, 11).
We have conducted various studies using mainly the Toxinometer®. The advantages of the Toxinometer® include its wide calibration range and the possibility of data reevaluation (when using LaboSoft-ET). In addition, there is no time constraint for the operator; stopping the reaction is not necessary once the measurement has been started. This can be a huge advantage for those performing the measurements. One advantage that is not well-known is that small turbidity of sample which doesn't change after the measurement has begun do not affect the measurement results because Tg determination is based on the principle of measured time until the ratio of transmitting light intensity reaches a threshold level.
References
- Guideline on Validation of the Limulus Amebocyte Lysate Test as an End-product Endotoxin Test for Human and Animal Parenteral Drugs, Biological Products, and Medical Devices. FDA (1987).
- Jorgensen, J.H. and Alexander, G.A. : Appl. Environ. Microbiol., 41, 1316-1320 (1981).
- Jorgensen, J.H. and Reichler, A.S. : J. Parenter. Sci. Technol., 36, 96-98 (1982).
- Oishi, H. et al. : Journal of the Pharmaceutical Society of Japan, 105, 300-303 (1985).
- Oishi, H. et al. : J. Parenter. Sci. Technol., 39, 194-200 (1985).
- Oda, Y. et al. : Kyushu Pharmacy Bulletin, 41, 29-38 (1987).
- Agui, W. et al. : J. Antibact. Antifung. Agents, 16, 313-322 (1988).
- Fujiwara, H. et al. : Journal of the Pharmaceutical Society of Japan, 110, 332-340 (1990).
- Tsuchiya, M. et al. : Japanese Journal of Bacteriology, 45, 903-911 (1990).
- Yokota, M. et al. : J. Biochem. Biopys. Methods, 18, 97-104 (1989).
- Kambayashi, J. et al. : J. Biochem. Biophys Methods, 22, 93-100 (1991).




