SPE Columns for Metal (Elemental) Capture and Recovery "Presep® PolyChelate"
Fujifilm Wako offers the Presep® series which is solid-phase extraction columns packed with a variety of packing agents for use in the pretreatment of food, environmental, and pharmaceutical component analysis.
Presep® PolyChelates are syringe-type columns for metal (elemental) capture and recovery, filled with a packing agent with a long-chain aminocarboxylic acid group as the chelating functional group. Compared to general SPE column, filled IDA (iminodiacetic acid) chelating resins, Presep® PolyChelate can capture and recover many elements (metals) with higher efficiency over a wide pH range. It can also efficiently capture and recover metal oxoacids (Mo, V, W) under acidic to neutral conditions without recovering alkali metals or alkaline earth metals. Presep® PolyChelate can be used for extraction and concentration of trace metals in seawater, river water, wastewater, and biological samples, as well as for removal of matrix in measurements by atomic absorption and ICP emission spectrometry.
Features
- Capable of capturing and recovering many metals (elements) with high efficiency over a wide pH range!
- Efficient capture and recovery of metal oxoacids (Mo, V, W) under acidic to neutral conditions without recovery of alkali metals and alkaline earth metals!
- Can be used as a SPE column in the separation and concentration method by chelating resin in JIS K 0102 "testing methods for industrial wastewater!"
Correlation Between pH and Recovery of 62 Elements When Using Presep® PolyChelate1)
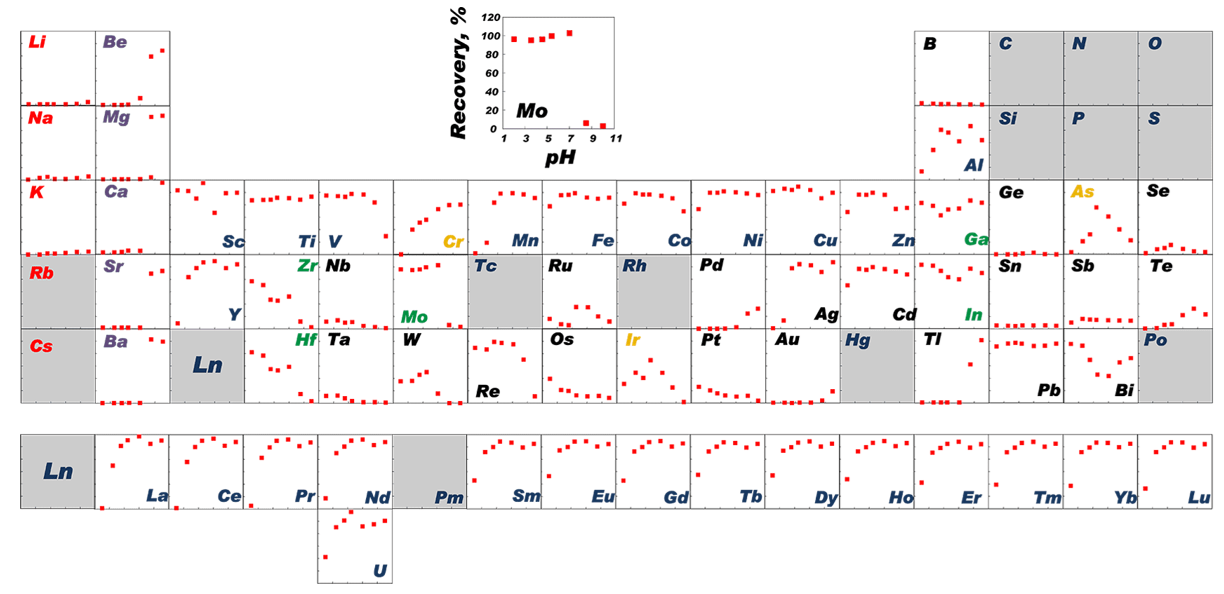
PerkinElmer Optima 3000 DV ICP-AES, cross-flow nebulizer and a Scott-type spray chamber
1) Provided by Prof. Kagaya, Faculty of Engineering, University of Toyama
Example of Solid-phase Extraction Operation
Example of solid-phase extraction operation*
pH Adjustment of the Sample
- 0.1 mol/L CH3COONH45 mL/100 mL
- pH Adjust using HNO3 or NH3 solution.
Loading*1
Flow rate: 3~10 mL/min.
*1 For samples with high solid content, perform solid-phase extraction after filtration through an appropriate filter.
*2 Used to remove alkaline earth metal elements from samples captured under alkaline conditions.
Elution
- 1~3 mol/L HNO33~5 mL
Analysis
* These conditions are only example and may require further study depending on the sample.
Instruction for use and handling
- Products must be conditioned prior to use. Drying the packing agent during conditioning may result in loss of reproducibility.
- Add a buffer solution that does not interact with elements (metals) such as ammonium acetate before pH adjustment of the sample solution.
- When passing the sample solution, reduce or increase the pressure to maintain a constant flow rate.
- To avoid adsorption and elution of elements (metals), please refrain from using metal utensils. Use glass or plastic utensils that have been thoroughly rinsed with nitric acid.
Example of Measurement of Elements in Treated Wastewater and Surface Seawater
Solid-phase extraction operation2)
pH Adjustment of the Sample
- 0.1 mol/L CH3COONH45 mL/100 mL
- pH Adjust using HNO3 or NH3 solution.
Elution
- 1~3 mol/L HNO33~5 mL
Analysis
Recovery rate2)
✔ Trace metal oxoacids (Mo, V) in seawater containing a large amount of alkali metals can be captured and recovered efficiently!
| Elements | Treated wastewater*1 (n=3) | Surface seawater*2 (n=3) | ||
|---|---|---|---|---|
| Detection (μg/L) | Recovery rate*3 (%) | Detection (μg/L) | Recoveryrate*3 (%) | |
| Cd | 0.2±0.02 | 93±2.9 | (0.03±0.004) | 96±1.7 |
| Co | <0.033 | 92±3.7 | (0.02±0.012) | 96±1.5 |
| Cu | 1.1±0.13 | 104±3.5 | 0.77±0.017 | 112±3.2 |
| Fe | 33±3.3 | 114±13.8 | 1.3±0.05 | 94±3.2 |
| Mn | 871±75 | 64±9.0 | Not measure | Not measure |
| Mo | 458±9.3 | 98±1.7 | 7.4±0.07 | 100±4.5 |
| Ni | 11±0.2 | 101±4.9 | 0.46±0.017 | 100±1.8 |
| Pb | <0.22 | 94±2.7 | 0.09±0.052 | 104±2.0 |
| V | <0.22 | 92±3.1 | 1.3±0.05 | 96±2.5 |
| Zn | 11±3.1 | 83±2.1 | 2.5±0.24 | 102±2.2 |
*1 Sample volume: 200 mL (pH 5.5)
*2 Sample volume: 300 mL (pH 4.0)
*3 Internal standard solution (5 μg of each element) added to the solution
2) Kagaya, S., Maeba, E., Inoue, Y., Kamichatani, W., Kajiwara, T., Yanai, H., Saito, M. and Tohda, K.: Talanta., 79, 146 (2009).
Product List
- Open All
- Close All
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



