Y-27632 (dihydrochloride) -ROCK Inhibitor
Y-27632 is a selective and potent inhibitor of ROCK (Rho-associated coiled-coil forming kinase/Rho binding kinase).Y-27632 has a variety of activities including vascular smooth muscle contraction through the ROCK signaling (Ki = 140 nmol/L p160ROCK). This product has been reported to suppress death of human ES/iPS cells during cell dispersion and to improve cell viability after cryopreservation.
We provide four types of Y-27632 products. You can select according to the purpose of uses.
| Y-27632 Products | Size | Availability/Price |
|---|---|---|
| Y-27632 (GMP-compliant) | 5 mg | |
| 25 mg | ||
| Y-27632, MF | 5 mg | |
| 25 mg | ||
| CultureSure® Y-27632 | 1 mg | |
| 5 mg | ||
| 25 mg | ||
| 100 mg | ||
| CultureSure® 10mmol/L Y-27632 Solution, Animal-derived-free | 300 μL | |
| 1 mL |
Overview
Y-27632 is a cell-permeable small molecule that selectively and potently inhibits Rho-associated coiled-coil containing protein kinases (ROCK1 and ROCK2). By competitively binding the ATP catalytic site of ROCK enzymes, Y-27632 effectively suppresses their kinase activity (ROCK1 Ki = 220 nM; ROCK2 Ki = 300 nM) (Ishizaki et al.,2000; Davies et al.,2000). This selective inhibition modulates cytoskeletal dynamics, cell adhesion, and apoptosis pathways, making Y-27632 a valuable tool in cellular biology and regenerative medicine research.
Key Applications of Y-27632
Maintenance and Self-Renewal of Stem Cells
Enhances Cell Survival:
Y-27632 significantly improves survival rates of human embryonic stem (ES) cells after single-cell dissociation by preventing apoptosis triggered by cell detachment (anoikis). This effect increases cloning efficiency and facilitates expansion of ES cultures (Watanabe et al., 2007).
Supports Embryoid Body Formation
When used in forced-aggregation protocols, Y-27632 enhances the formation of embryoid bodies, promoting three-dimensional differentiation potential (Ungrin et al., 2008).
Improves Post-Thaw Viability
The compound protects cryopreserved single human ES cells from apoptosis after thawing, resulting in higher recovery and viability (Martin-Ibañez et al., 2008, Li et al., 2008, Li et al., 2009).
Neural Precursor Survival
In embryonic stem cell-derived neural precursor cells, Y-27632 blocks apoptosis following cell dissociation and transplantation, supporting successful cell engraftment (Koyanagi et al., 2008).
Promotes Neurite Outgrowth
Y-27632 regulates microtubule remodeling to regulate neurite outgrowth (Chen et al., 2013).
Reprogramming and Lineage Conversion
Y-27632 serves as a critical component in direct lineage reprogramming protocols. In combination with other small molecules (such as CHIR99021, RepSox, Forskolin, SP600125, Gö6983, and Valproic Acid), it facilitates the conversion of fibroblasts into mature neurons, broadening its utility in regenerative medicine and cellular engineering (Hu et al., 2015).
Differentiation Protocols
During initiation of differentiation, particularly in human ES cell monolayers, Y-27632 enhances cell survival, enabling more efficient and reproducible differentiation outcomes (Rezania et al., 2014). Additionally, by modulating actomyosin contractility in the stem cell niche, Y-27632 influences cell shape and mechanical cues that regulate stem cell lineage commitment (McBeath et al., 2004).
Y-27632 also improves viability and proliferation in other cell types, such as human keratinocytes cultured in vitro, enabling their efficient immortalization and long-term culture (Chapman et al., 2010).
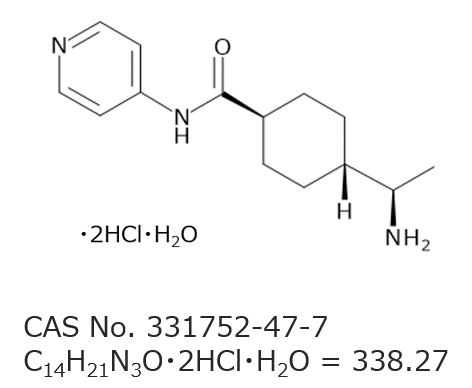
Chemical Information for Y-27632
| CAS Number | 331752-47-7 |
|---|---|
| Chemical Formula | C14H21N3O・2HCl・H2O |
| Molecular Weight | 338.27 |
| Purity (HPLC) |
Y-27632 (GMP-compliant) : ≧99.5% Y-27632, MF : ≧98.0% CultureSure® Y-27632 : ≧98.0% |
| Chemical Name | (R)-(+)-trans-N-(4-Pyridyl)-4-(1-aminoethyl)cyclohexanecarboxamide Dihydrochloride Monohydrate |
Product Information
Y-27632 (GMP-compliant)
This product is complied with ICH-Q7 (GMP for APIs) and is intended as a raw material for the commercial production of regenerative medicine products. It has been tested for viable count, as well as bacterial endotoxins, mycoplasma contamination, and residual solvent.
- GMP compliant
- Process validation analytical validation carried out
- No animal-derived ingredients are used as raw materials
Y-27632, MF
This is an ISO9001-compliant product developed for the commercial manufacturing of regenerative medicine products. It has been tested for viable count, as well as bacterial endotoxins and mycoplasma contamination.
The management and manufacturing standards have been established internally.
- ISO9001 compliant
- Reduction of contamination risk through the use of dedicated equipment for manufacturing culture media additives
- Process validation, analytical validation, and cleaning validation carried out
- No animal-derived ingredients are used as raw materials
CultureSure® Y-27632
This product was developed for basic research in drug discovery and regenerative medicine. It has been tested for bacterial endotoxins and mycoplasma contamination.
- ISO9001 compliant
- No animal-derived ingredients are used as raw materials
CultureSure® 10 mmol/L Y-27632 Solution, Animal-derived-free
This product is a 10 mmol/L aqueous solution of Y-27632. It has been tested for sterility, as well as bacterial endotoxins and mycoplasma contamination. It has been 0.1 μm filter-sterilized and can be added directly to the culture medium.
- ISO9001 compliant
- No animal derived-ingredients are used as raw materials
Protocols
Here is a protocol for passaging, freezing, and thawing human ES/iPS cells (single cells), using our product Y-27632.
Applications
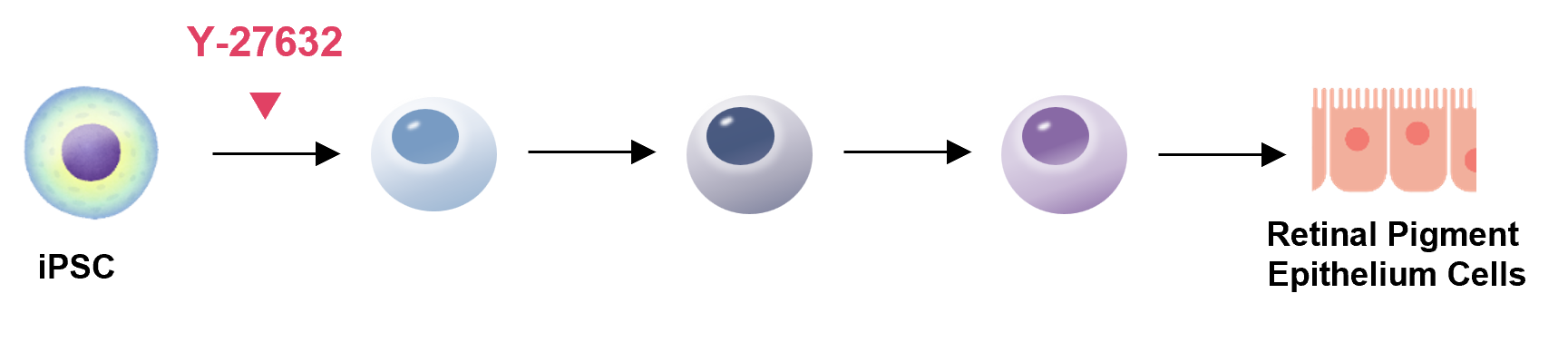
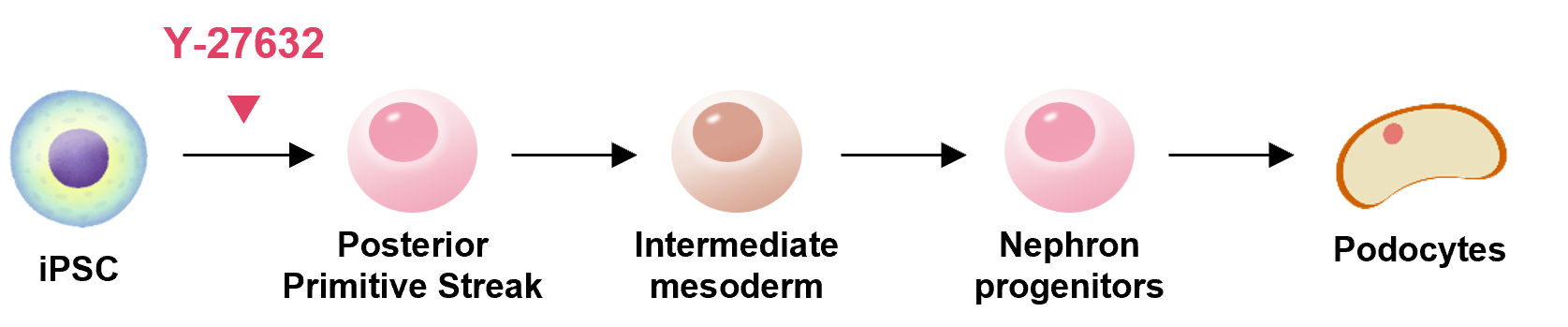
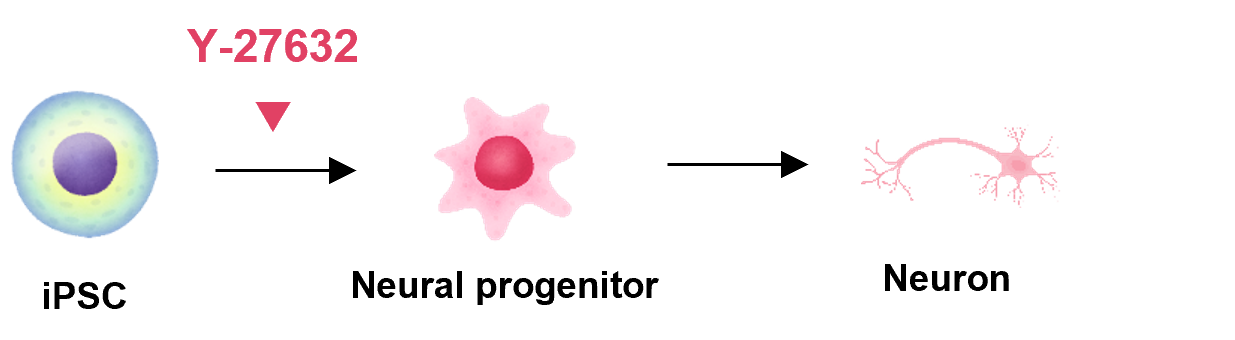
Y-27632 is used for effective differentiation induction of iPS/ES cells.
Based on the latest findings reported in the literature, we introduce examples of the usage steps for our products.
Nerves
References
Retina
Lung
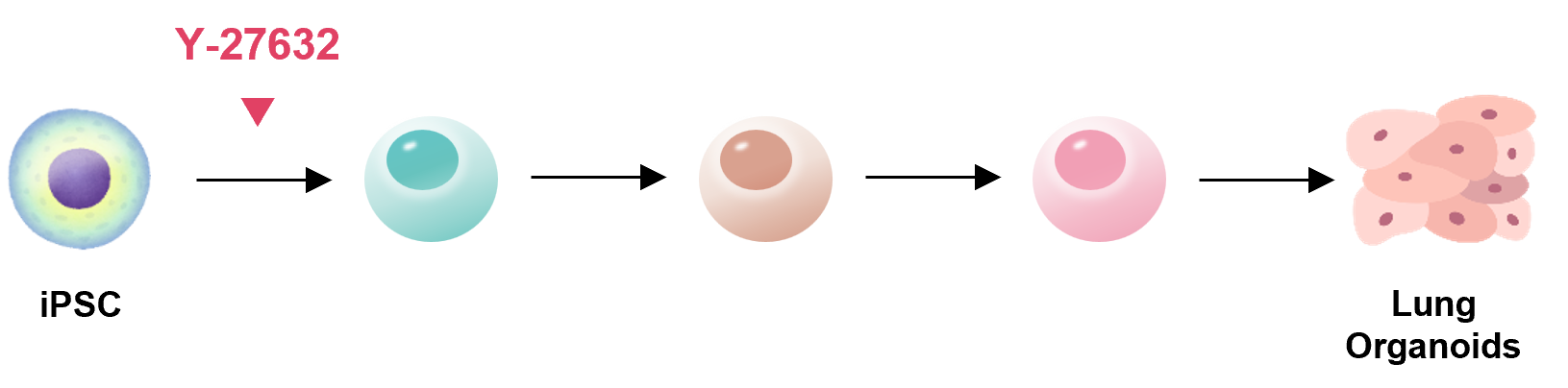
References
Kidney
Y-27632 Comparison Chart
| ICH-Q7 | ISO9001 | Sterility test |
Viable count test |
Animal- derived- free |
Bacterial Endotoxin test |
Mycoplasma test |
Powder/ Liquid |
References* | |
|---|---|---|---|---|---|---|---|---|---|
| Y-27632 (GMP-compliant) | ✓ | ✓ | - | ✓ | ✓ | ✓ | ✓ | Powder | - |
| Y-27632, MF | - | ✓ | - | ✓ | ✓ | ✓ | ✓ | Powder | |
| CultureSure® Y-27632 | - | ✓ | - | - | ✓ | ✓ | ✓ | Powder | |
| CultureSure® 10mmol/L Y-27632 Solution, Animal-derived-free |
- | ✓ | ✓ | - | ✓ | ✓ | ✓ | Liquid Sterilized with filter |
* Click to move to the PubMed Central® search results.* Click to move to the PubMed Central® search results.
Preparing Stock Solutions for Y-27632
The powder Y-27632 product has been confirmed to be soluble in water. After dilution, filter sterilize before use. Refer to the protocol at the top of this page for general usage in ES/iPS cell culture.
Amount of solvent when preparing solution
| Y-27632 (powder) | ||||
|---|---|---|---|---|
| 1 mg | 5 mg | 10 mg | ||
| Concentration | 1 mmol/L | 2.956 mL | 14.781 mL | 29.562 mL |
| 5 mmol/L | 0.591 mL | 2.956 mL | 5.912 mL | |
| 10 mmol/L | 0.296 mL | 1.478 mL | 2.956 mL | |
| 50 mmol/L | 0.059 mL | 0.296 mL | 0.591 mL | |
| Solution Volume | |||||
|---|---|---|---|---|---|
| 0.3 mL | 1 mL | 5 mL | 10 mL | ||
| Concentration | 1 mmol/L | 0.101 mg | 0.338 mg | 1.691 mg | 3.383 mg |
| 5 mmol/L | 0.507 mg | 1.691 mg | 8.457 mg | 16.914 mg | |
| 10 mmol/L | 1.015 mg | 3.383 mg | 16.914 mg | 33.827 mg | |
| 50 mmol/L | 5.074 mg | 16.914 mg | 84.568 mg | 169.135 mg | |
Resources and Publications
Literature and Education
- Map of Culture Compounds and Cytokines
- Small Molecules Catalog
- On-demand webinar:Impact of ROCK inhibitor in suspension aggregate culture of iPS cells
- FUJIFILM Wako Life Science Webinar Series
Publications
| CultureSure® 10mmol/L Y-27632 Solution, Animal-derived-free |
|---|
| Noriko, H. et al.: Exp Ther Med, 24, 539 (2022). Formation of three-dimensional cell aggregates expressing lens-specific proteins in various cultures of human iris-derived tissue cells and iPS cells |
| Suguru, Y. et al.: iScience, 25, 103657 (2021). A Genetic modification that reduces ON-bipolar cells in hESC-derived retinas enhances functional integration after transplantation |
| Masatoshi, H. et al.: Cell Rep Med, 6, 101847 (2025). A first-in-human clinical study of an allogenic iPSC-derived corneal endothelial cell substitute transplantation for bullous keratopathy |
| Y-27632 MF |
|---|
| Ryosuke, I. et al.: Bio Protoc, 15, e5449 (2025). Isolation and Co-culture of Paneth Cells and Intestinal Stem Cells. |
| Tomokazu, F. et al.: Sci Rep, 13, 9655 (2023). A ROCK inhibitor suppresses the transforming growth factor-beta-2-induced endothelial-mesenchymal transition in Schlemm's canal endothelial cells |
| Marie, P. et al.: Mater Today Bio, 14, 100232 (2022). Brain microvascular endothelial cells derived from human induced pluripotent stem cells as in vitro model for assessing blood-brain barrier transferrin receptor-mediated transcytosis |
| Ratklao, S. et al.: iScience, 25, 104130 (2022). Single-cell RNA sequencing identifies a migratory keratinocyte subpopulation expressing THBS1 in epidermal wound healing |
| Y-27632 ( GMP-compliant ) |
|---|
| Hanna, V. et al.: Nat Commun, 15, 5558 (2024). Ube3a unsilencer for the potential treatment of Angelman syndrome |
Glossary
GMP
Good Manufacturing Practice (GMP) is a standard for manufacturing and quality control of pharmaceutical drugs, etc., and should be observed by manufacturers of pharmaceutical drugs, etc.
The GMP standards are based on the following three principles:
- Minimizing human errors during manufacture
- Preventing contamination and quality deterioration of pharmaceutical products
- Designing a system for high quality assurance
ICH-Q7 (GMP for APIs)
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is an international initiative to create guidelines on pharmaceutical regulations from scientific and technical perspectives by joint efforts of regulatory authorities and representatives of the pharmaceutical industry.
Among these guidelines, ICH-Q7 (GMP for APIs (Active Pharmaceutical Ingredients)) outlines the standard practices for manufacturing management and quality control related to active pharmaceutical ingredients.
ISO9001
ISO stands for the International Organization for Standardization, a non-governmental organization. The standards established by the ISO are referred to as ISO standards. These are internationally agreed-upon criteria established by experts, covering various areas such as product manufacturing, process management, service provision, and material supply.
ISO 9001 is a standard set out for quality management systems. Many companies and organizations are certified to ISO 9001 to ensure that their customers consistently receive good quality products and services.
White Paper
Product List
- Open All
- Close All
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.
The prices are list prices in Japan.Please contact your local distributor for your retail price in your region.