WakoVAC MDCK
Fujifilm Wako promotes our proprietary media product lines under WakoVAC, manufactured under GMP-compliant system and scalable to commercial volumes. WakoVAC MDCK is designed to maximize the growth of MDCK cell and its virus production, and built to support for large manufacturing.
Wako Proprietary Media
Products developed under WakoVAC brand is taking a comprehensive approach to meet the key requirements for cell culture products in the field of life science. WakoVAC MDCK is targeted to support optimal and stable expansion of MDCK cells while retaining viability and target protein expression.
Raw Material Supply
Animal components free raw materials are used in WakoVAC MDCK. All raw materials are documented and traceable. All raw materials used are standardized to a certain specification, scalable, and provided from validated suppliers. WakoVAC MDCK contains non-animal hydrolysate.
Industrial Applications
The intended use of WakoVAC (GMP-compliant manufactured) is for research, process development, large-scale manufacturing/commercialization. WakoVAC is scalable to commercial volumes for industrial application. Both liquid and powder are available. Flexible packaging is also available upon request. Wako provides complete workflow support from media optimization service to regulatory support at the time of IND/NDA.
Performance
WakoVAC MDCK includes multiple prototypes formulated to support MDCK cell types. It is designed to maximize and consistent MDCK cell growth, viability and sustains high productivity.
Features
- Free of animal component. *Includes non-animal hydrolysate.
- Compatible with microcarriers.
- Adaptation not required.
- Customizable to suit your cells
- Optimized for small- to large-scale vaccine production.
- Compatible with a range of scales of production from research to industry.
- GMP-compliant manufacturing and materials management including raw material changes.
Product Information
| Form | Powder or liquid |
|---|---|
| Packaging | Based on needs |
| Shelf-life | 12 months (powder, data under collection) |
| Storage | Protect from light at 2 – 10℃ * The attached supplement (vial) should be stored at -20℃ or below |
Performance
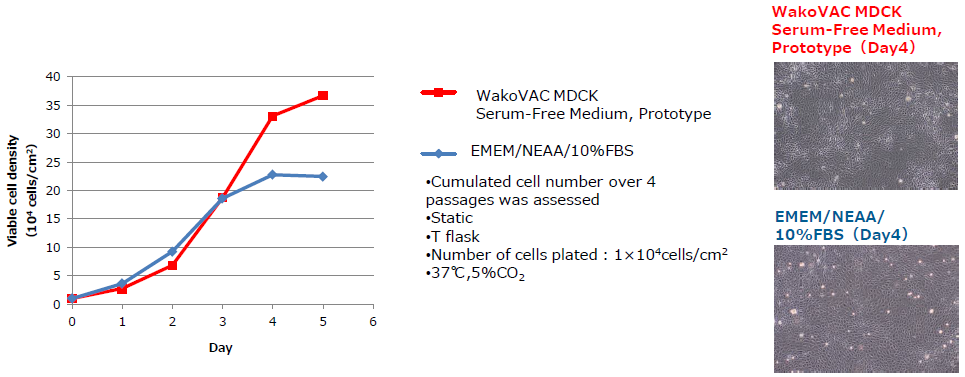
Cell growth is superior to classical medium that contains FBS.
Cell Growth
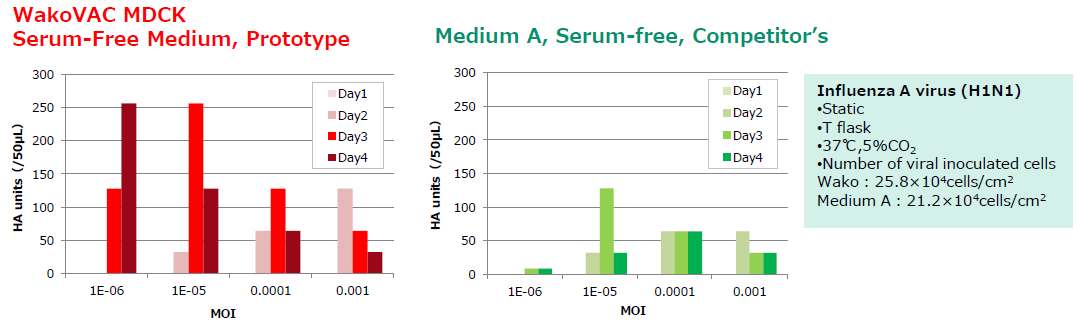
Virus productivity greater than or equal to that of competitor' serum-free medium.
Virus Productivity
Smooth adaptation in switching from serum-free medium or serum-based medium.
- Switch from a commercially available serum-free medium
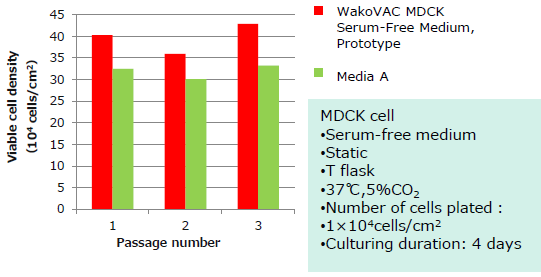
- Switch from a serum-containing medium
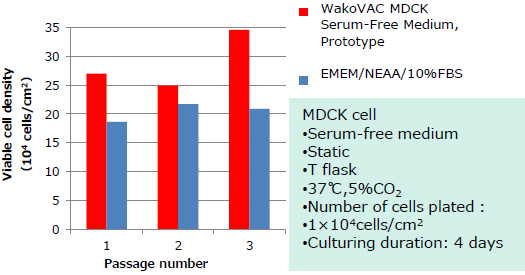
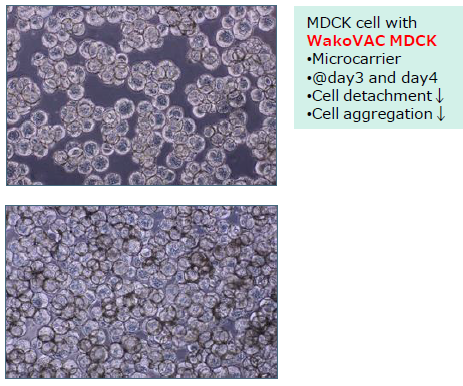
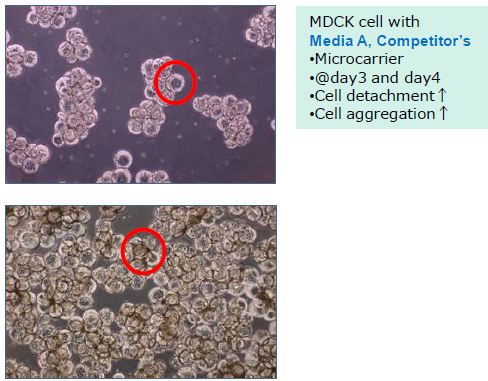
Compatible with cell culture using microcarriers.
WakoVAC MDCK prevents cell detachment and cell aggregation on microcarriers during cell culture.
Media Optimization Service
This product is custom manufactured and supplied upon request. Please use the inquiry form below for sample requests, quotes, and questions. The person in charge will contact you for further information.
Service Flow
- 1. Inquiry
- 2. Making test sample at FUJIFILM Wako
- 3. Sample testing at your site
- 4. Feedback of the test result
- 5. Customization
- 6. Fix formulation
- 7. Place order
- 8. Ship the media
| Note: -We respond to flexible small scale media demand with non-GMP samples formulated with the same quality raw materials used in GMP level. COS (testing includes appearance, pH and osmo) is available upon request. -The shipping cost of the sample will be decided after consultation. -A confidentiality agreement will be concluded upon request. |
Pharmaceutical Raw Materials
Product List
- Open All
- Close All
This is a made-to-order product. Please visit Media Optimization Service.
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.