Lithium Cation Endohedral Fullerene
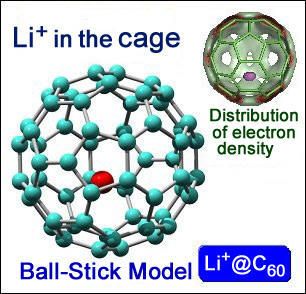 Lithium endohedral fullerene is a novel nanomaterial displaying a very high ionic conductivity (unlike empty fullerene). From this conductivity, the material is likely to have wide applications dye-sensitized and organic solar cells.
Lithium endohedral fullerene is a novel nanomaterial displaying a very high ionic conductivity (unlike empty fullerene). From this conductivity, the material is likely to have wide applications dye-sensitized and organic solar cells.
FUJIFILM Wako markets products made by Idea International, which is the world's first company to succeed in mass production of Lithium endohedral fullerene (Li+@C60).
Application1
A new solution to highly efficient solar cells
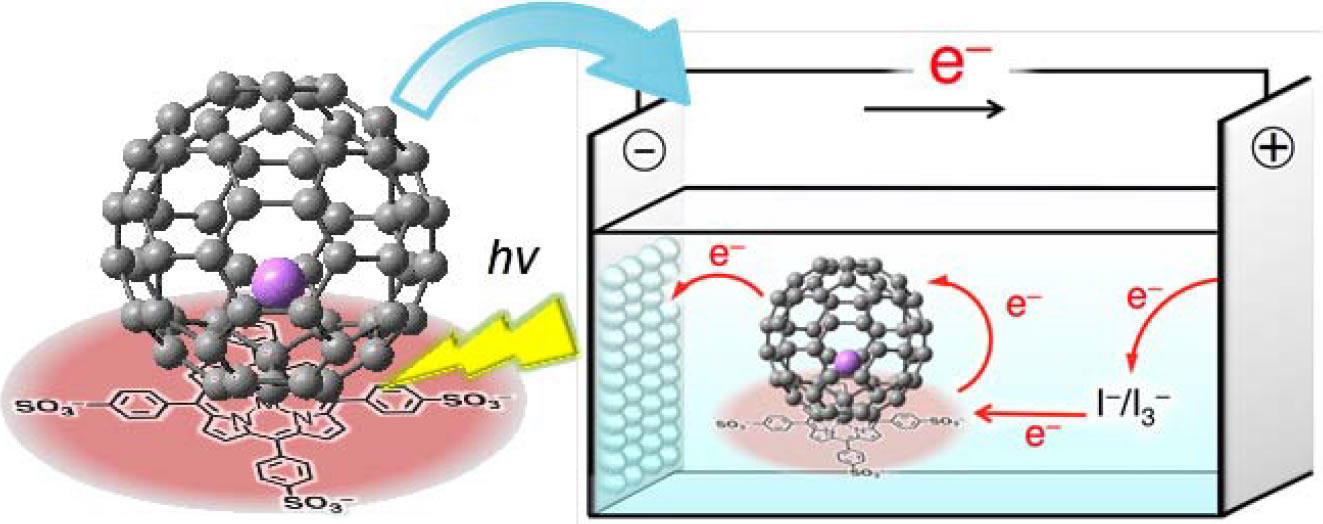
Li+@C60 provides long lasting separation of electron charge. Li+@C60-sulphonated porphyrin supramolecular nanoclusters support increased photoelectricalchemical performance of photovoltaic cells. Photoelectrochemical solar cells composed of supramolecular nanoclusters of lithium encapsulated fullerene and zinc sulfonated meso-tetraphenylporphyrin exhibit significant performance enhancement as compared with the reference system containing only a single component.
References
- Ohkubo, Y. Kawashima and S. Fukuzumi, "Strong supramolecular binding of Li+@C60 with sulfonated meso-tetraphenylporphyrins and long-lived photoinduced charge separation", Chem. Commun., 48, 4314-6 (2012)
- Ohkubo, Y. Kawashima, H. Sakai, T. Hasobe and S. Fukuzumi, "Enhanced photoelectrochemical performance of composite photovoltaic cells of Lii+@C60- sulphonated porphyrin supramolecular nanoclusters ", Chem. Commun., 49, 4474-6 (2013)
Application2
Li+ ion is encapsulated inside C60 (Fullerene).
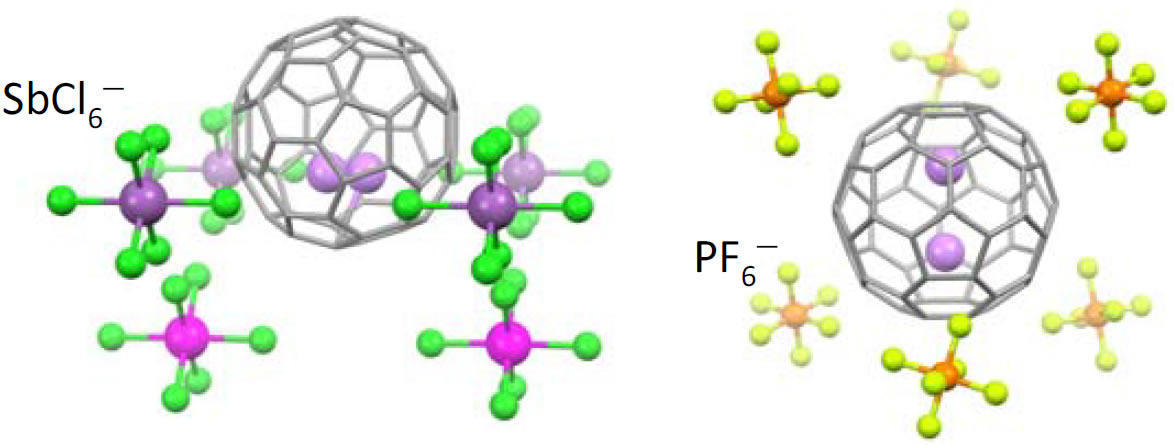
Li+@C60 reacts with various anions and forms salts (e.g. SbCl6- and PF6-), which are useful in switch and sensor applications. This property can be utilized for sensors and switches.
References
- Aoyagi, E. Nishibori, H. Sawa, K. Sugimoto, et al., "A layered ionic crystal of polar Li@C60 superatoms", Nature Chemistry, 2, 678-83 (2010).
- Aoyagi, Y. Sado, E. Nishibori, et al., "Rock-Salt-Type Crystal of Thermally Contracted C60 with Encapsulated Lithium Cation", Angew. Chem. Int. Ed., 51, 3377-81 (2012).
Application3
Higher ion mobility in organic solvent
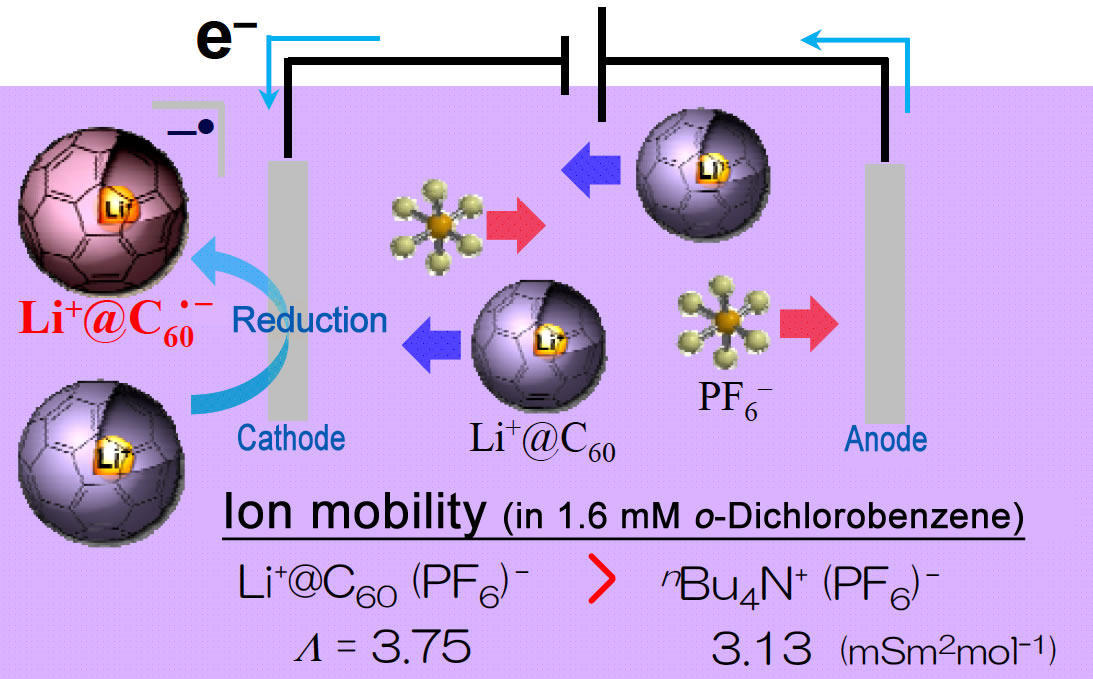
Li+@C60- is produced in the reaction above, and is electrically neutral. Because Li+@C60·PF6- shows higher mobility inside organic solvents than n-Bu4N+·PF6-, which is widely used as electrolyte, many electro-chemical applications of Li+@C60 are envisaged. In fact, Li+@C60·-, a radical anion, is selectively produced when Li+@C60-·PF6- is reduced.
References
- Ueno, K. Kokubo, Y. Nakamura, K. Ohikubo, et al., "Ionic conductivity of [Li+@C60](PF6-) in organic solvents and its electrochemical reduction to Lii+@C60·-", Chem. Commun., 49, 7376-8 (2013).
Product List
- Open All
- Close All
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



