Fluorescent-labeled rBC2LCN
rBC2LCN (AiLecS1) is a recombinant lectin produced by expressing the N-terminal domain of BC2L-C, a lectin derived from Burkholderia cenocepacia, in Escherichia coli. rBC2LCN binds with high affinity to H type 3 (Fucα1-2Galβ1-3GalNAc), a mucin-type O-glycan present on a protein called podocalyxin. Because podocalyxin is found on the surface of human ES/iPS cells, it has been reported that rBC2LCN can be employed as a marker for undifferentiated human ES/iPS cells.
Labeled with a fluorochrome, this product can simply be added to the culture solution to analyze live undifferentiated ES/iPS cells. It is compatible with both cell staining and flow cytometry. No cytotoxicity has been detected in continuous culture for several days in the presence of this product.
The product lineup offers two types of fluorescent labels: green (fluorescein isothiocyanate) and red (Cy5 spectrum).
Features
- Staining can be achieved by simply adding the product to the medium
- Can stain live cells clearly without the need for fixation
- Low cytotoxicity allows culture of stained cells
- Recognizes sugar chains on cell surfaces in the same manner as Tra-1-60, Tra-1-81, SSEA-3 and SSEA-4
- Compatible with cell staining and cytometry
Product outline
- Passed sterility testing (filtration sterilization performed with a 0.1 μm filter)
- PBS (-) solution
- Working dilutions
Live Cell Imaging 1:100 - 1,000
Flow Cytometry 1:100 - 1,000 - Excitation and emission wavelengths
| Excitation | Emission | |
|---|---|---|
| rBC2LCN-FITC | 495 nm | 520 nm |
| rBC2LCN-635 | 634 nm | 654 nm |
Instruction for use
Cell staining
Live Cell Imaging
- Prepare human ES/iPS cells being cultured.
- Add 1-2 μL of fluorescent-labelled rBC2LCN per 1 mL of medium.
- Incubate for 30 minutes at 37°C in 5% CO2.
- Replace the medium containing rBC2LCN with either fresh medium or HBSS (+).
- Perform fluorescence microscopy.
Staining of fixed cells
- Prepare human ES/iPS cells being cultured.
- Remove the medium and wash the cells with HBSS (+).
- Add 4% paraformaldehyde (PFA) and let stand for 10-20 minutes at room temperature.
- Aspirate the PFA and wash the cells three times with D-PBS (-).
- Add D-PBS (-).
- Add 1-10 μL of fluorescent-labelled rBC2LCN per 1 mL of D-PBS (-).
- Incubate for 30 minutes at 37°C in 5% CO2.
- Replace the existing D-PBS(-) with fresh D-PBS(-).
- Perform fluorescence microscopy.
《 Flow cytometry 》
- Prepare human ES/iPS cells being cultured
- Use cell dissociation solution to break colonies down into single cells.
- Transfer the cell dissociation solution to a test tube and centrifuge at 1,000 rpm for 3 minutes.
- Remove supernatant and suspend cells with FCM Buffer (flow cytometry buffer consisting of D-PBS(-) or HBSS(-), and may also include 10 mmol/L EDTA and 1% BSA). Centrifuge and discard the supernatant.
- Suspend cells in FCM Buffer to a concentration of 5×106 cells/mL.
- Add 1-10 μL per 1 mL of cell suspension.
- Let stand for 30 minutes in the dark at room temperature.
- Centrifuge at 1,000 rpm for 3 minutes and discard the supernatant,
- Suspend cells with FCM Buffer, Centrifuge and discard the supernatant again.
- Resuspend cells with an appropriate amount of FCM Buffer.
- Perform flow cytometry.
〔Precautions〕
- rBC2LCN staining will persist for 2 to 3 days.
- Medium containing serum may generate enhanced background signals.
- When conducting cell sorting by flow cytometry, add Y-27632 to a final concentration of 10 μmol/L during the cell dissociation or FCM Buffer steps.
Product notes
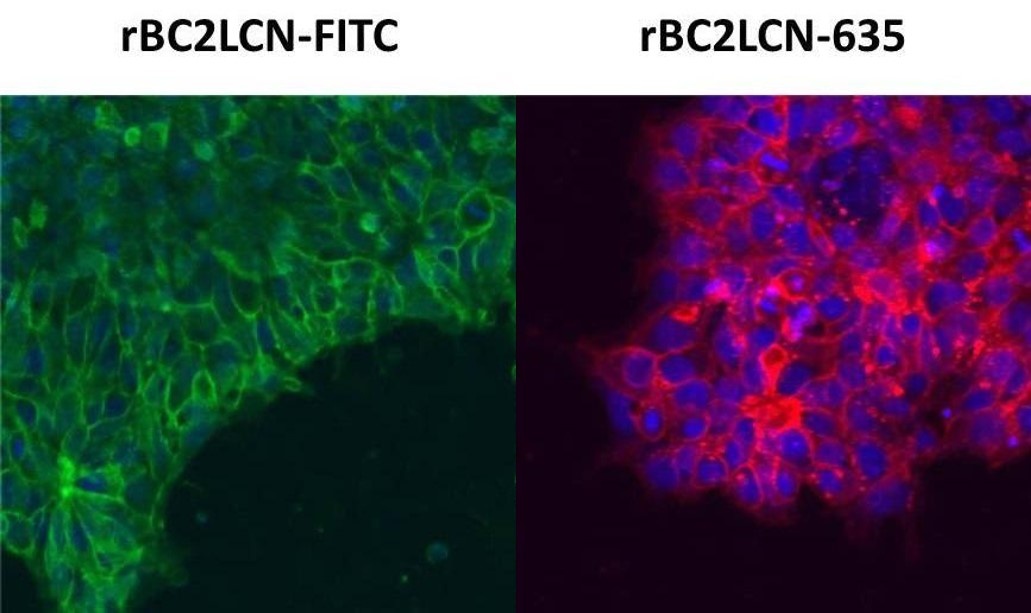
Live cell imaging of human iPS cells
Human iPS cell line 201B7 was stained with rBC2LCN-FITC, rBC2LCN-635 and Hoechst 33342 (without fixation).
(Dilution ratio 1:1,000)
Human iPS cell line 201B7 was stained with rBC2LCN-FITC, Tra-1-60, Tra-1-81 and SSEA-4, after which images of the stained cells were confirmed 2 hours later (without fixation).
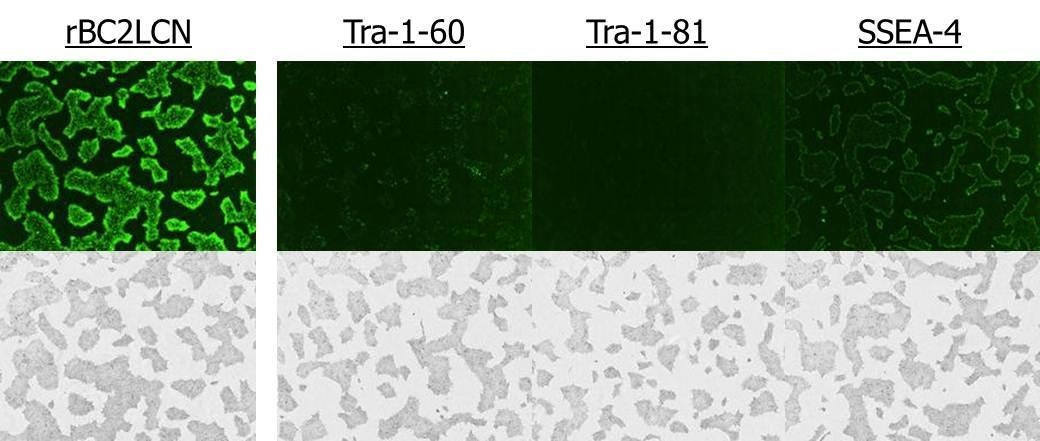
(Dilution ratio 1:100)
- rBC2LCN rendered stronger live cell imaging compared to Tra-1-60 and Tra-1-81.
- rBC2LCN staining persisted vividly up to two days after medium replacement.
Live cell imaging of human ES cells
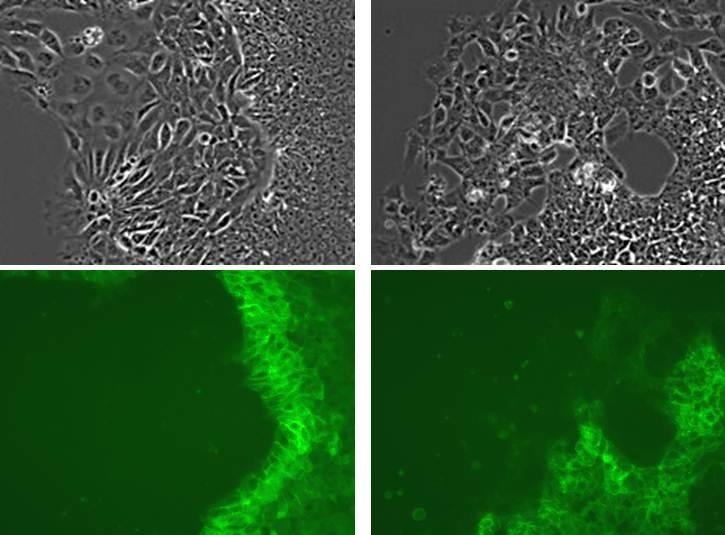
Human ES cell line WA01 was stained with rBC2LCN-FITC (without fixation). Unable to stay undifferentiated, a portion of the cells underwent differentiation. These images show how rBC2LCN-FITC stains undifferentiated cells but not differentiated one.
<Data provided by Yasuko Onuma and Yuzuru Ito, Stem Cell Engineering Research Group, Biotechnology Research Institute for Drug Discovery, National Institute of Advanced Industrial Science and Technology>
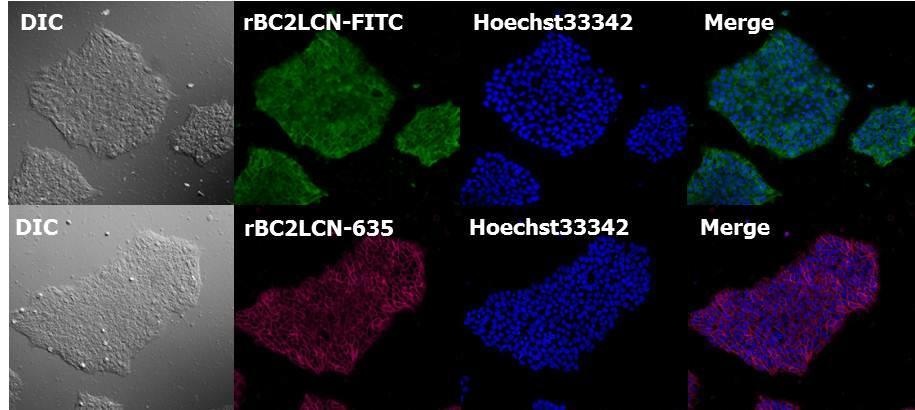
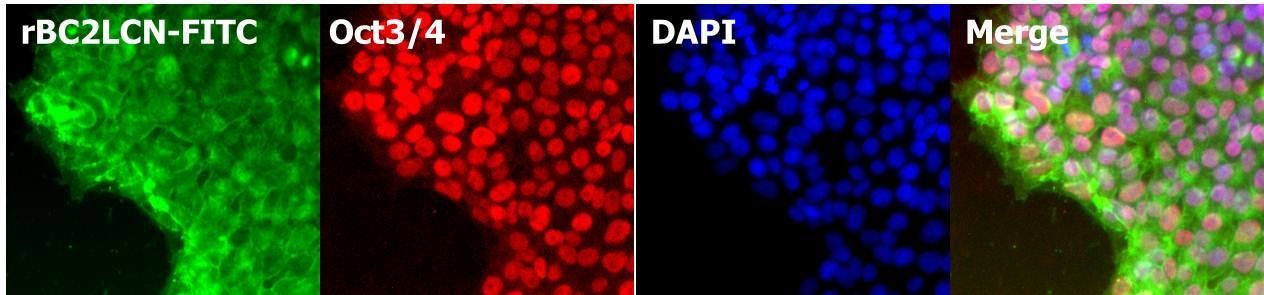
Human iPS cell staining after fixation
After fixing human iPS cell line 201B7 with paraformaldehyde, cell staining was performed with rBC2LCN and DAPI.
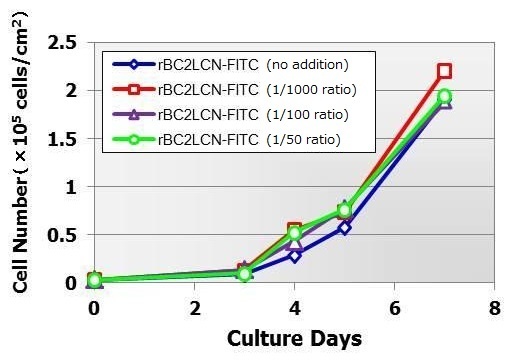
Cytotoxicity evaluations of human iPS cells
rBC2LCN-FITC was added to cultures of human iPS cell line 201B7 at ratios of 1/1,000, 1/100 and 1/50 of the culture solution. Cell culture was allowed to continue. It was consequently found that cell proliferation was similar for all samples regardless of rBC2LCN-FITC concentration.
〔Cell line〕
Human iPS cell line 201B7
〔Medium〕
StemSure®hPSC MediumΔ + 35ng/ml bFGF
〔Coating〕
Matrigel® hESC-Qualified Matrix
〔Seeding density〕
4×104cells/well (with a 12 well plate)
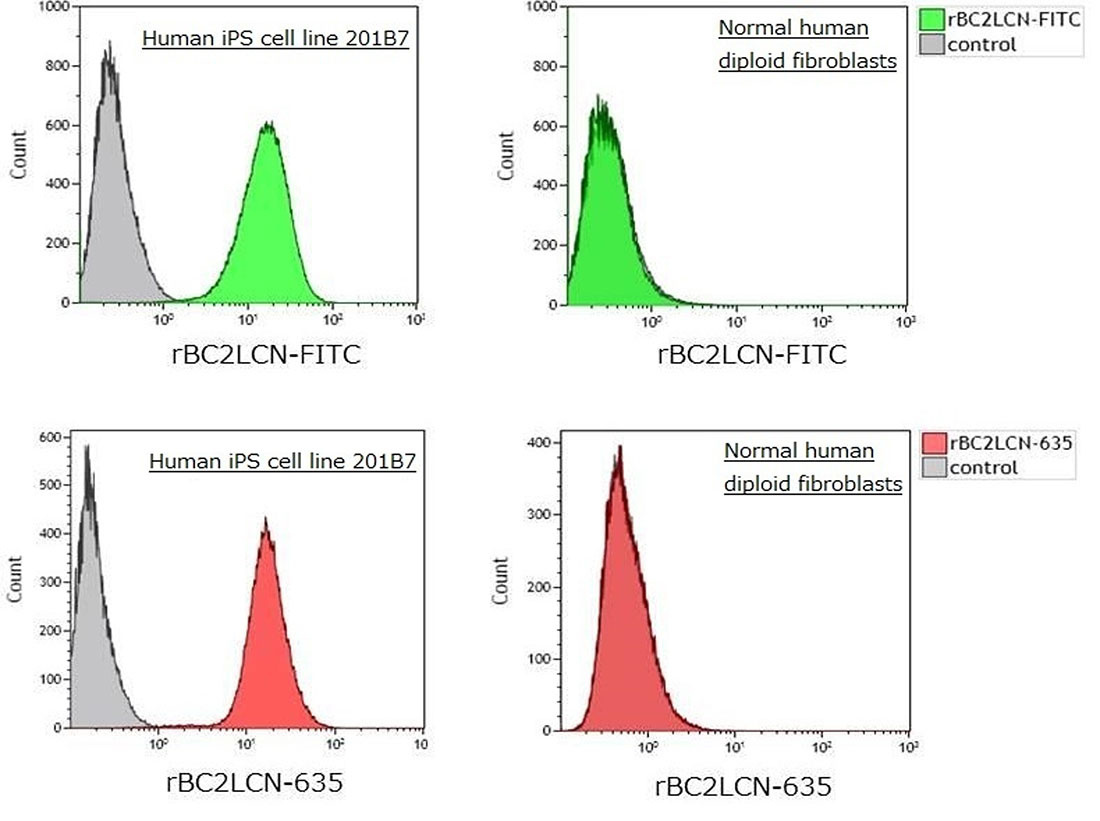
Separation of human iPS cell separation with flow cytometry
After human iPS cell line 201B7 and normal human diploid fibroblasts were stained with rBC2LCN-FITC, rBC2LCN-547 and rBC2LCN-635, flow cytometry was performed. As a result, it was possible to separate undifferentiated human iPS cells from differentiated normal human diploid fibroblasts.
References
Onuma, Y., et al.: Biochem. Biophys. Res. Commun., 431, 524, (2013).
Tateno, H., et al.: Stem Cells Transl. Med., 2, 265, (2013).
Tateno, H., et al.: Sci. Rep., 4, 4069, (2014).
Product List
- Open All
- Close All
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



