Y-27632, MF
Y-27632, MF was registered in the Drug Master File (MF) in Japan as additives for culture medium in September 2015. We ensure high stability and quality of the products by validating the manufacturing and analytical processes and implementing change control strategies.
GMP products comming soon!
FUJIFILM Wako will provide the small molecules produced through GMP based on ICHQ7 in 2021.
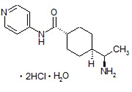
What is Y-27632?
Selective and potent ROCK inhibitor
Y-27632 plays various roles including contraction of vascular smooth muscle cells via ROCK signaling pathways. Furthermore, it suppresses the cell death of ES/iPS cells at cell dispersion, and that it improves cell survival after cryopreservation.
CAS RN®. 331752-47-7
C14H21N3O・2HCl・H2O=338.27
Product Specification
| Requirement | Specification |
|---|---|
| Appearance | White to pale yellow, crystalline powder to powder |
| Assay (HPLC) | ≧98.0% |
| Solubility in water | To pass test |
| Specific rotation [α]D20(c=1.0,CH3OH) | +2.0~+10.0゜ |
| Bacterial endotoxins※ | <0.25EU/mg |
| Viable count test※ | 20CFU/g以下 |
| Mycoplasma test※ | To pass test |
- Tests for mycoplasma, endotoxin, and viable bacteria are performed for every lot as part of standard tests; however, the results are not required for MF registration.
- Registration in the Drug Master File (MF) does not indicate that the product was tested for its quality and safety by the Ministry of Health, Labor and Welfare (Pharmaceuticals and Medical Devices Agency).
References
Uehata, M., et al. : Nature, 389, 990 (1997).
Sakamoto, K., et al. : J. Pharmacol. Sci., 92, 56 (2003).
Nishimaru, K., et al. : J. Pharmacol. Sci., 92, 424 (2003).
Watanabe, K., et al. : Nat. Biotechnol., 25, 681 (2007).
Martin-Ibanez, R., et al. : Hum. Reprod., 23, 2744 (2008).
Claassen, DA., et al. : Mol. Reprod. Dev., 76, 722 (2009).
Kawamata, M., et al. : Proc. Natl. Acad. Sci. USA., 107, 14223 (2010).
Ito, H., et al. : Liver Int., 32, 592 (2012).
Katsuda,T., et al. : Cell Stem Cell, 20, 41 (2017).
Okae, H., et al. : Cell Stem Cell, 22, 50 (2018).
Ogawa, K., et al. : Sci. Rep., 8, 3615 (2018).
Ayabe, H., et al. : Stem Cell Reports., 11, 306 (2018).
Product List
- Open All
- Close All
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



