SuperSep™ DNA
SuperSep™ DNA is non-denaturing polyacrylamide gel for double-stranded DNA. The acrylamide concentration and the gel cross-linking density are optimized for the separation of low-molecular-weight DNA. This product is suitable for the separation of 20 to 200 bp DNA.
Product specifications
| Acrylamide concentration | 15% |
|---|---|
| Plate size | 100 x 100 x 3 (mm) |
| Gel size | 90 x 85 x 1 (mm) |
| Number of wells | 17 wells |
| Well volume | 25 μL/well |
Recommended running buffers
- 1×TBE Buffer
- 1×Tris-Glycine Buffer (25 mmol/L Tris, 192 mmol/L Glycine)
Example:
5 x TBE (Product Number 318-90041)
10 x Tris-Glycine Buffer (Product Number 201-18601)
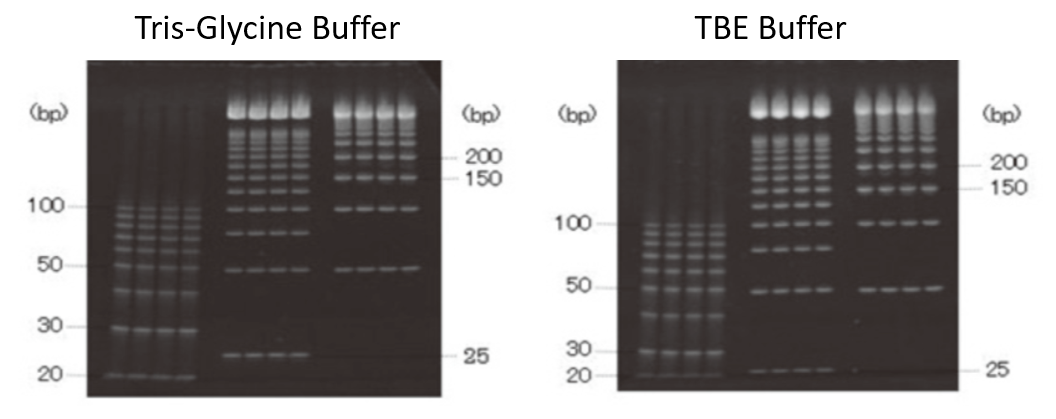
Example of use 1: Separation of low-molecular-weight DNA
Using SuperSep™ DNA and Tris-Glycine Buffer, 20 to 200 bp DNA was clearly separated (left). Using TBE Buffer, 30 to 200 bp DNA was clearly separated (right). SuperSep™ DNA can be used to excise PCR products up to 200 bp in length, especially from small RNA cloning gels.
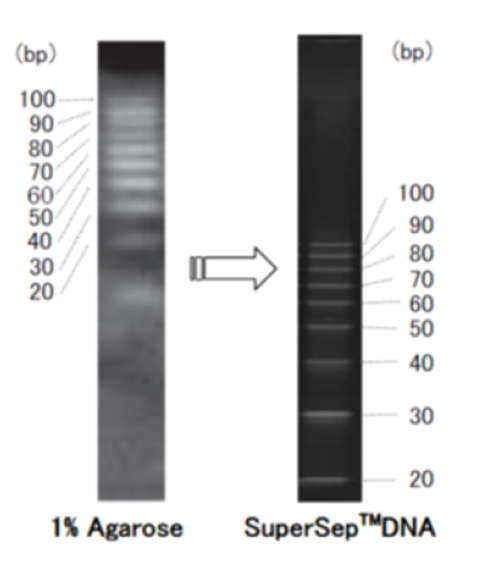
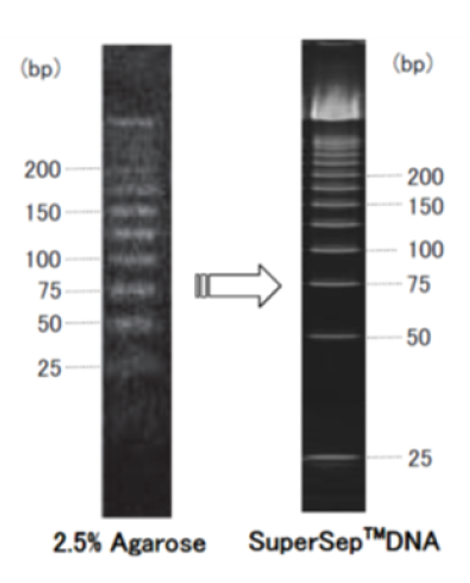
Example of use 2: Comparison with agarose gel
When low-molecular-weight DNA is electrophoresed, bands tend to be smeared on agarose gel, but clear separation is obtained on SuperSep™ DNA.
-
Comparison with 1% agarose gel
-
Comparison with 2.5% agarose gel
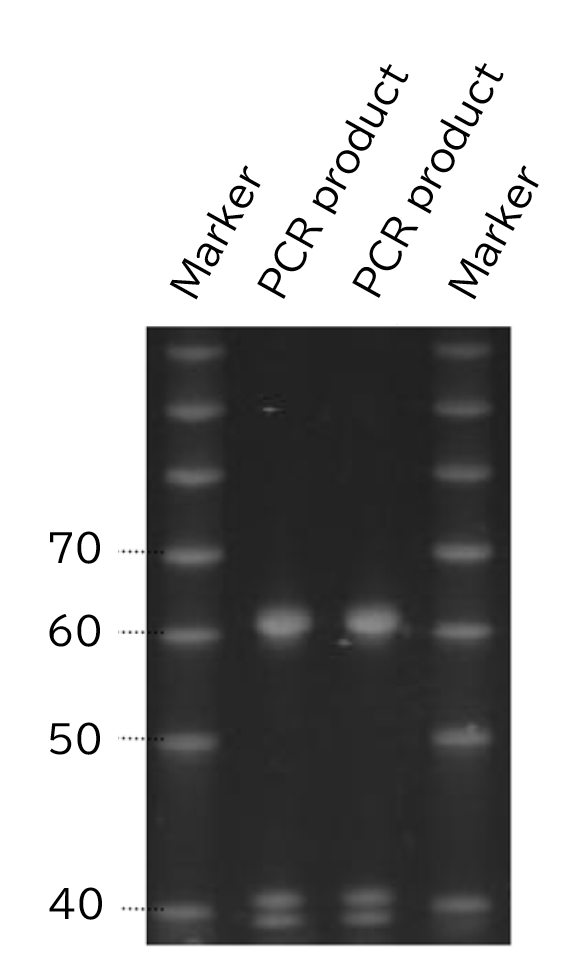
Example of use 3: PCR product electrophoresis
Preprocessing protocol
When the PCR product is directly electrophoresed, bands may be smeared due to contamination with low-molecular-weight proteins. In such circumstances, the following preprocessing is recommended:
- After PCR, add and mix an equal volume of mixture of phenol, chloroform, and isoamyl alcohol (25:24:1).
- Centrifuge the mixture at 4°C and 14,000 rpm (18,800 × g) for 5 minutes, and transfer the top layer to a microtube.
- Add the following to the microtube and mix: 1 μL of Ethachinmate, 10 mol/L Ammonium acetate in one-fourth volume of the aqueous layer collected, and 99.5% ethanol in twice the total volume.
- Centrifuge the mixture at 4°C and 14,000 rpm (18,800 × g) for 15 minutes, and remove the supernatant. Wash the precipitate twice with 70% ethanol.
- After drying at no more than 45°C, dissolve the precipitate in 10 μL of sterile water, and mix the solution with 2 μL of 6 × Loading Buffer Triple Dye.
- Apply 6 μL of the mixture to the gel.
Electrophoresis conditions
Buffer for electrophoresis::25 mmol/L Tris, 192 mmol/L Glycine
Current : 30 mA x 60min (constant current)
Staining : Ethidium bromide staining × 15 minutes
Product List
- Open All
- Close All
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.
The prices are list prices in Japan.Please contact your local distributor for your retail price in your region.