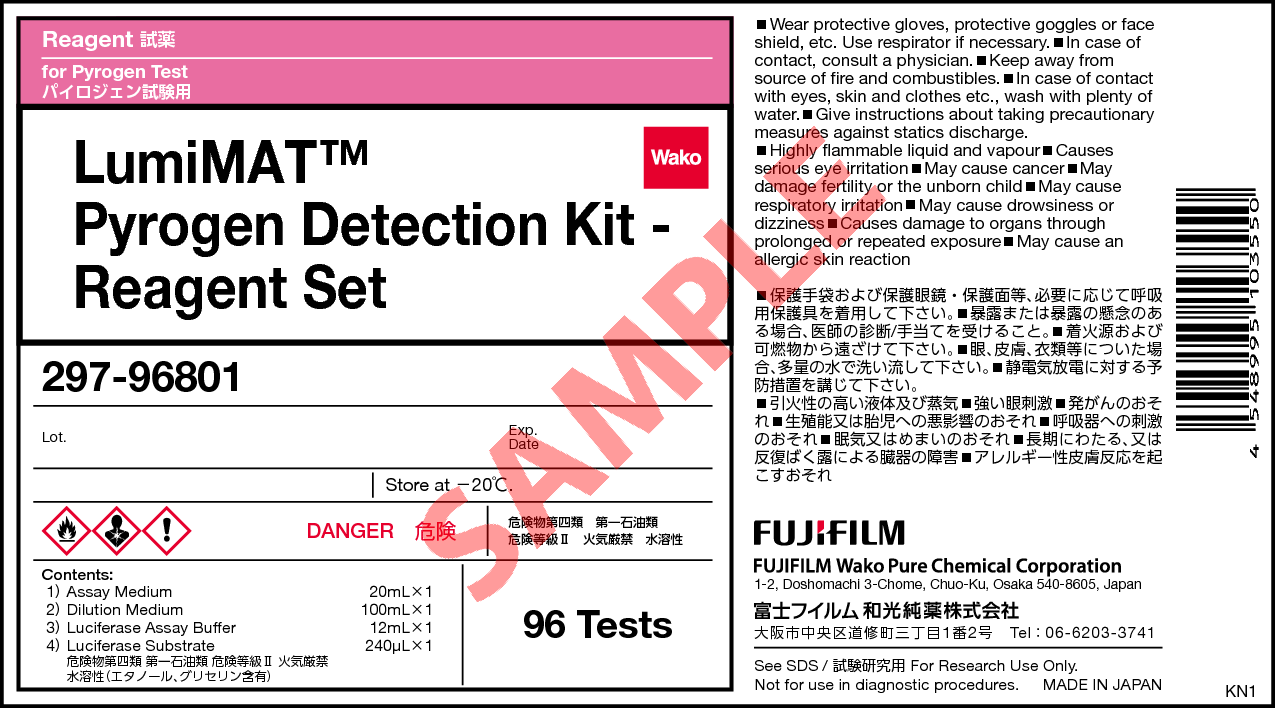
LumiMAT™ Pyrogen Detection Kit - Reagent Set
- for Pyrogen Test
- Manufacturer :
- FUJIFILM Wako Pure Chemical Corporation
- Storage Condition :
- Keep at -20 degrees C.
- GHS :
-
- Structural Formula
- Label
- Packing
- SDS
|
Comparison
|
Product Number
|
Package Size
|
Price
|
Availability
|
Certificate of Analysis
|
Purchase |
|---|---|---|---|---|---|---|
|
|
|
96Tests
|
|
In stock in Japan |
※Check availability in the US with the distributor.
Document
Kit component
96 tests
| Assay medium | 20 mL |
|---|---|
| Dilution medium | 100 mL |
| Luciferase assay buffer | 12 mL |
| Luciferase substrate | 240 μL |
Overview
LumiMAT™ is a Monocyte Activation Test (MAT) to detect pyrogens. Pyrogen is a generic term for substances that cause body temperature rise in animals and humans. Parenteral drugs and medical devices which get in contact with patient's blood system must be free from pyrogens. The first developed test to detect a wide range of pyrogens was Rabbit Pyrogen Test (RPT). In RPT, samples to be tested are intravenously administered to rabbits and monitor the body temperature. Since RPT has problems with reproducibility, accuracy, and cost, Limulus Amebocyte Lysate (LAL) reagent has been widely used as the replacement. However, LAL reagent cannot detect non-endotoxin pyrogens, and RPT is still used in case LAL reagent is not appropriate. MAT is an in vitro pyrogen test developed as an alternative of RPT and it can detect not only endotoxin but also non-endotoxin pyrogens.
| RPT | LAL | MAT | ||
|---|---|---|---|---|
| Pyrogens | Endotoxin (derived from Gram-negative bacterial cell walls) |
|||
| Non-endotoxin Pyrogens (derived from Gram-positive bacteria, viruses, fungi etc.) |
Not-detectable | |||
Assay principle
MAT is used to detect pyrogens that activate human monocytic cells to release inflammatory cytokines.
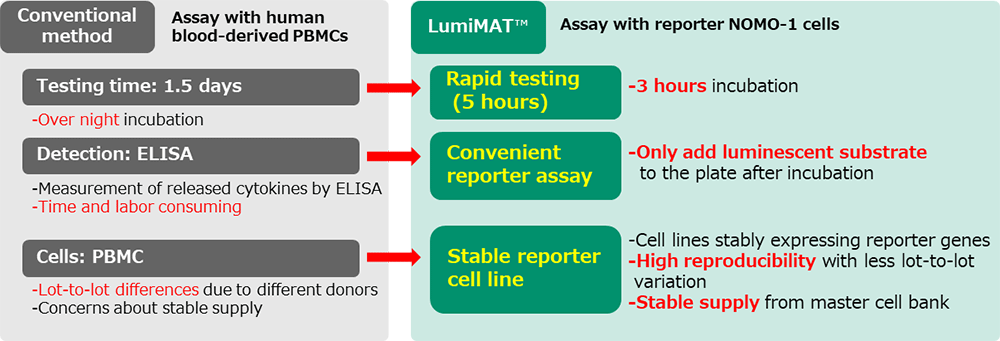
Conventional MAT
Culture PBMCs (peripheral blood mononuclear cells) and samples to be tested in microwell plate. Cytokines released from monocytic cells by exposure to pyrogens are measured by ELISA to detect pyrogens in the samples.
LumiMAT™
LumiMAT™ uses a monocytic cell line NOMO-1 in which a luciferase reporter gene is introduced to express luciferase protein in response to NF-κB signals activated by exposure to pyrogens. Because the expressed luciferase generates luminescence by reacting with the substrate, luminescence can be detected by a luminescence microplate reader to detect pyrogens in the samples.
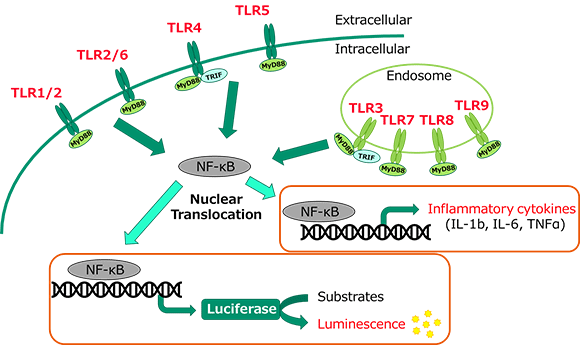
Features of LumiMAT™
Assay Flow
Both LumiMAT™ Pyrogen Detection Kit - Cells and LumiMAT™ Pyrogen Detection Kit - Reagent Set are necessary for assay.
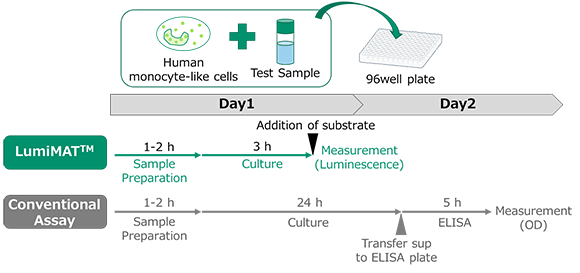
Add samples to be tested or standard endotoxin into microplate and add reporter cells and incubate for 3 hours. After the incubation add luminescent substrate to measure luminescence with a plate reader.
Compared to ELISA, this is a simple one-plate assay that suppresses inter-test variations. In addition, results can be obtained in about 5 hours, which is significantly shorter than the 1.5 days required by the conventional method.
Standard curve
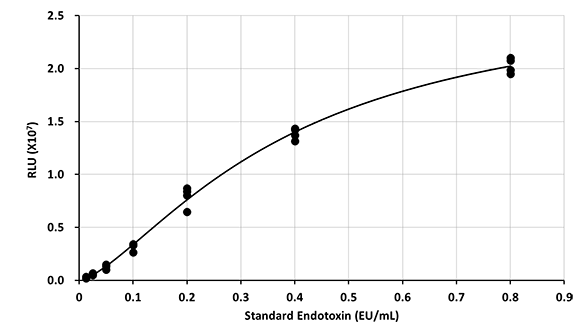
EU:Endotoxin Unit
Create standard curve using reference standard endotoxin and calculate the concentration of pyrogens as Endotoxin Equivalents (EE)/mL.
Standard curve range: 0.0125 - 0.8 EE/mL
How to use LumiMAT™
Experimental Data
Reproducibility
Accuracy and precision were measured with three different lots of the LumiMAT cells (n=4, 3 assays for each lot). Six concentrations of standard endotoxin (USP-RSE) were spiked. Measured the concentrations of endotoxin (EU/mL) of the spiked samples from the standard curve and calculated accuracy and precision.
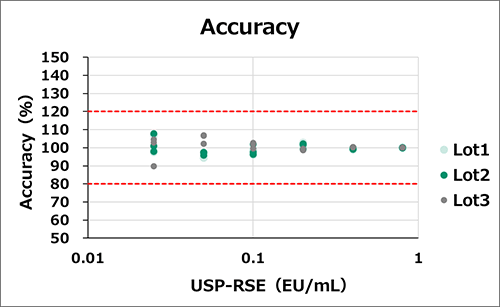
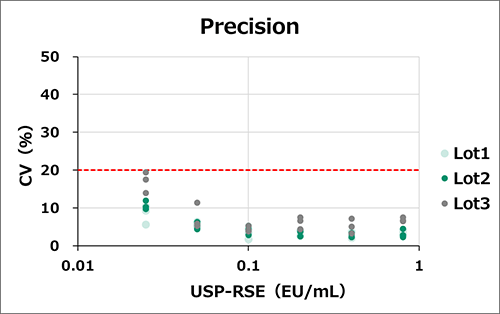
Everly lots had high reproducibility.
Accuracy [measured value/true value] : within ±10%, Precision [CV of measured value] : within 20%
Reactivity to Non-Endotoxin Pyrogens (NEPs)
NEPs which react with each Toll-like receptor (TLR) were added at several concentrations, and the concentration of each NEP was calculated as EE/mL from the standard curve.
Y axis: EE/mL, X axis: Spiked concentration of NEP
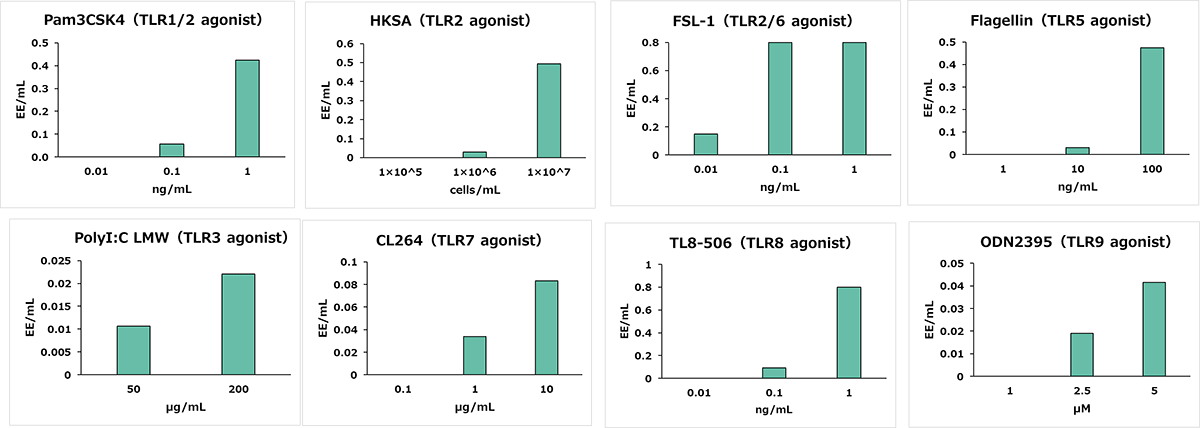
All tested NEPs were detected.
Tests for interfering factors
1. Spike recovery test using pharmaceutical products.
Standard endotoxin was added to each drug product and the recovery rate was calculated.
| Drug | MVD* | Fold-dilution | Spike recovery (%) n=3 | Interference** | ||
|---|---|---|---|---|---|---|
| 0.1 EU/mL | 0.4 EU/mL | 0.8 EU/mL | ||||
| Albumin 25%I.V. 5 g/20 mL |
480 | 10 | 121.6 | 98.9 | 97.5 | N |
| 100 | 134.8 | 119.4 | 110.3 | N | ||
| 400 | 109.5 | 110.3 | 108.0 | N | ||
| Aciclovir 25 mg/mL |
500 | 10 | 67.5 | 63.7 | 50.2 | N |
| 100 | 89.7 | 87.8 | 82.3 | N | ||
| 400 | 96.0 | 95.6 | 99.2 | N | ||
| Epoetin Alfa 750 I.U./0.5 mL |
12000 | 100 | 99.3 | 103.1 | 105.2 | N |
| 500 | 92.9 | 100.5 | 102.6 | N | ||
| 1000 | 87.9 | 98.0 | 98.9 | N | ||
| Romosozumab 105 mg/1.17 mL |
10200 | 100 | 109.9 | 110.5 | 112.6 | N |
| 500 | 94.9 | 94.3 | 98.6 | N | ||
| 1000 | 95.0 | 92.0 | 92.3 | N | ||
*MVD : Maximum Valid Dilution **No interference : 50 < Recovery(%) < 200
Recovery rate were within 50-200% for all four pharmaceuticals.
2. Spike recovery test using pharmaceutical raw materials
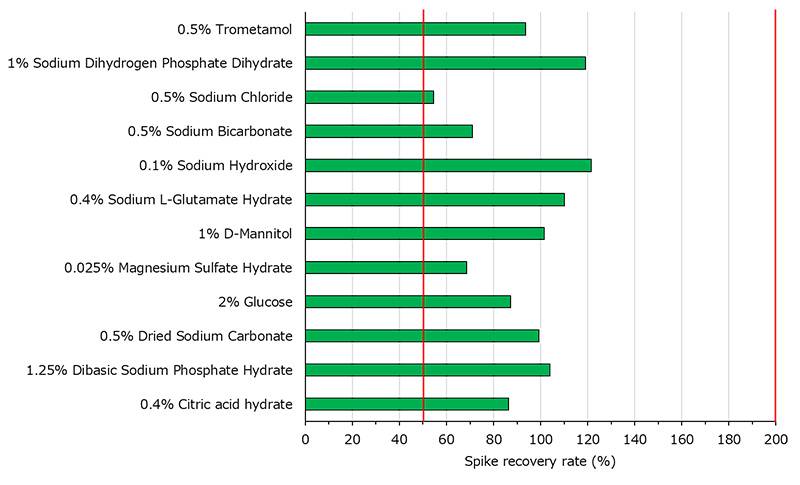
LumiMAT™ is applicable for a variety of samples.
Overview / Applications
Property
Manufacturer Information
Alias
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.