LBIS (TM) Human Apo B-48 ELISA Kit
- for Immunochemistry
- Manufacturer :
- FUJIFILM Wako Pure Chemical Corporation
- Storage Condition :
- Keep at 2-10 degrees C.
- GHS :
-
- Structural Formula
- Label
- Packing
- SDS
|
Comparison
|
Product Number
|
Package Size
|
Price
|
Availability
|
Certificate of Analysis
|
Purchase |
|---|---|---|---|---|---|---|
|
|
|
96Tests
|
|
In stock in Japan |
※Check availability in the US with the distributor.
Document
Kit component
96 tests
| Antibody-coated Plate | 1 plate |
|---|---|
| Human Apo B-48 Standard | 1 bottle |
| Buffer | 60 mL |
| Biotin-conjugated Antibody Solution | 100 µL |
| Peroxidase-conjugated Streptavidin Solution | 100 µL |
| TMB Solution | 12 mL |
| Stop Solution | 12 mL |
| Wash Solution (10x) | 100 mL |
| Plate Seal | 4 sheets |
Product Overview
Apolipoprotein B-48 (Apo B-48) is a structural protein specific to chylomicrons (CM), which transport exogenous lipids, primarily derived from food, to the liver and peripheral tissues. Apo B-48 is regarded as an optimal marker for monitoring postprandial exogenous lipid transport. By measuring Apo B-48, HDL cholesterol, and LDL cholesterol in the same sample, changes in exogenous and endogenous cholesterol levels can be assessed.
The LBIS™ Human Apo B-48 ELISA Kit is a sandwich ELISA kit designed to quantify human Apo B-48. This kit contains antibodies specific to Apo B-48, with cross-reactivity to Apo B-100 below the detection limit.
Kit Performance
| Calibration curve range | 2.5-160 ng/mL |
|---|---|
| Assay target | Apo B-48 |
| Analysis sample | Human serum/plasma (Citrate plasma cannot be used) |
| Sample volume | 50 μL (100-fold dilution) |
| Measurement duration | Approx. 2 hours 50 minutes |
| Wavelength | Primary wavelength 450 nm Reference wavelength 620 nm |
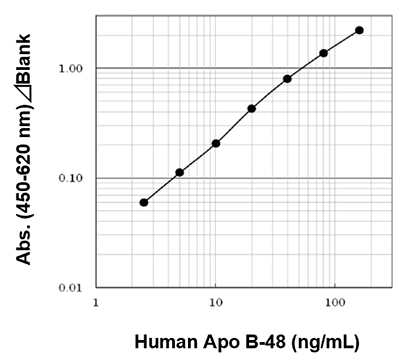
Example of Calibration Curve
Data
Repeatability (within run precision)
Using this product, quintuplicate measurements were performed on human serum (healthy donors, East Asian, males, 20–36 years old) at three concentrations to evaluate repeatability.
| n \ ID | ID1 (ng/mL) | ID2 (ng/mL) | ID3 (ng/mL) |
|---|---|---|---|
| 1 | 2.99 | 18.8 | 87.1 |
| 2 | 2.89 | 19.8 | 88.4 |
| 3 | 3.22 | 18.2 | 89.8 |
| 4 | 3.12 | 17.9 | 91.2 |
| 5 | 2.95 | 18.5 | 92.1 |
| mean | 3.03 | 18.6 | 89.7 |
| SD | 0.134 | 0.730 | 2.03 |
| CV(%) | 4.4 | 3.9 | 2.3 |
[Result]
The CV (%) ranged from 2.3% to 4.4%, indicating good repeatability.
Reproducibility (between run precision)
Using this product, measurements were performed on three separate days at three concentrations of human serum to evaluate reproducibility.
| Day \ ID | ID1 (ng/mL) | ID2 (ng/mL) | ID3 (ng/mL) |
|---|---|---|---|
| 1 | 2.85 | 28.2 | 79.8 |
| 2 | 2.95 | 29.9 | 80.1 |
| 3 | 3.01 | 25.2 | 75.9 |
| mean | 2.94 | 27.8 | 78.6 |
| SD | 0.0808 | 2.39 | 2.34 |
| CV(%) | 2.8 | 8.6 | 3.0 |
[Result]
The CV (%) ranged from 2.8% to 8.6%, indicating good reproducibility.
Dilution Linearity Test
Each human serum sample (healthy donors, East Asian, male, 20–36 years) was measured in duplicate at four concentrations following two-fold serial dilution to confirm linearity.
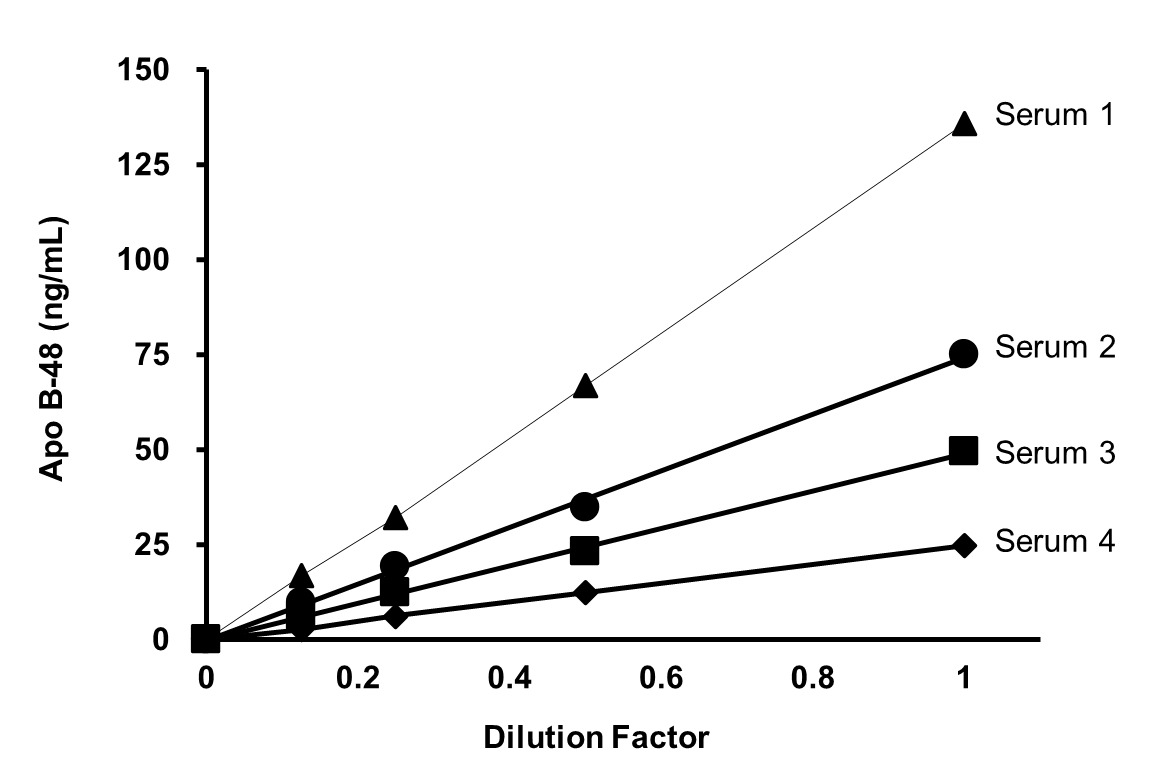
[Result]
All samples demonstrated good linearity within the measurement range.
Spiked Recovery Test
Each human serum sample (healthy donors, East Asian, male, 20–36 years) was spiked with the standard at three concentrations and measured in duplicate.
| Amount spiked (ng/mL) |
Measured value (ng/mL) |
Recovery volume (ng/mL) |
Recovery rate (%) |
|
|---|---|---|---|---|
| Serum 1 | 0.00 | 31.3 | - | - |
| 20.0 | 51.2 | 19.9 | 99.5 | |
| 40.0 | 70.1 | 38.8 | 97.0 | |
| 60.0 | 87.7 | 56.4 | 94.0 | |
| Serum 2 | 0.00 | 4.80 | - | - |
| 5.00 | 10.0 | 5.20 | 104 | |
| 10.0 | 15.3 | 10.5 | 105 | |
| 15.0 | 19.7 | 14.9 | 100 |
[Result]
Good recovery rate was confirmed.
Cross-Reactivity with Similar Proteins
Using this product, cross-reactivity was assessed by measuring structurally related proteins at each concentration.
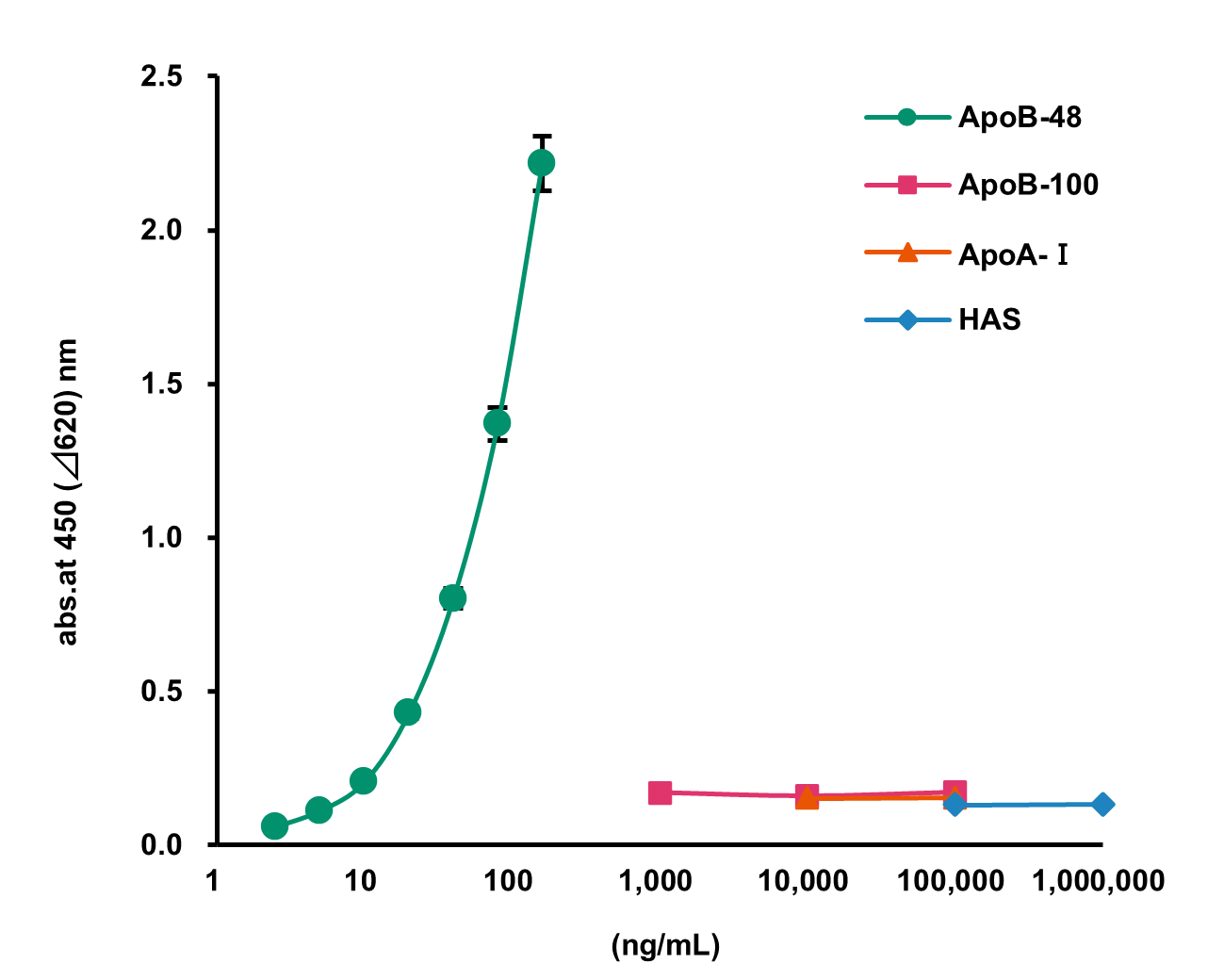
[Result]
Even at increasingly high concentrations, the measured values of Apo B-100, Apo A-I, and HAS (Human Albumin Solution) remained unchanged, confirming the absence of cross-reactivity.
Measurement of Apo B-48 in Human Serum
Sample: Human Serum (healthy donors, fasting, n=2)
| Sample No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | Mean | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measured value (µg/mL) |
3.59 | 5.66 | 5.86 | 6.19 | 6.56 | 4.63 | 4.16 | 8.12 | 5.58 | 2.69 | 2.83 | 4.11 | 2.88 | 5.28 | 3.05 | 2.65 | 4.69 | 4.23 | 4.6 | 1.54 |
The above measurements are presented as examples; actual values may vary depending on factors such as blood collection and sample storage conditions.
Application Data
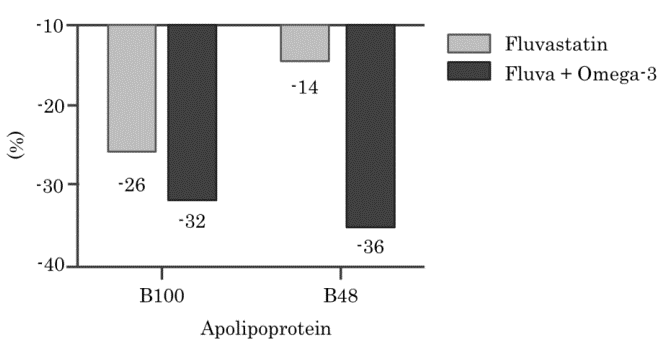
Changes in Plasma Apolipoprotein Levels Following Fluvastatin and Omega-3 Fatty Acid Administration1)
Patients with type 2 diabetes and hyperlipidemia were administered either 80 mg of fluvastatin alone or 80 mg of fluvastatin combined with 4 g of omega-3 fatty acids. Blood samples were collected after a 12-hour fasting period, and Apo B-100 and Apo B-48 levels were measured in each sample.
[Result]
Apo B-48 levels significantly decreased following the administration of fluvastatin and omega-3 fatty acids.
Changes in Apo B-100 and Apo B-48 levels relative to baseline
Created from reference 1)
References
- Valdivielso, P. et al.: Cardiovasc. Diabetol., 8, 1(2009).
Omega 3 fatty acids induce a marked reduction of apolipoprotein B48 when added to fluvastatin in patients with type 2 diabetes and mixed hyperlipidemia: a preliminary report
FAQ
About sample
- How should samples be stored after collection?
- Collect samples according to standard procedures and either measure them immediately or store them frozen at −35°C or below. Use test tubes or similar containers made of polypropylene (PP) or polyethylene (PE); glass containers are not suitable.
If refrigerated storage is unavoidable, add aprotinin to achieve a final concentration of 100–500 KIU/mL to prevent possible Apo B-48 degradation, and perform the assay within 2 days (KIU: Kallikrein Inhibitor Unit).
Frozen samples should be thawed immediately before measurement and mixed thoroughly. Our validation indicates that up to two freeze–thaw cycles do not affect assay results; however, avoid repeated freeze–thawing whenever possible. These recommendations should be regarded as general guidelines only, as sample characteristics and storage conditions may vary.
- Which anticoagulants are recommended for plasma samples?
- EDTA-2Na, EDTA-2K, and heparin are acceptable anticoagulants based on our validation. Citrate must not be used, as it affects assay results.
- How should samples be diluted?
- Dilute samples using the buffer provided with the kit in polypropylene (PP) or polyethylene (PE) tubes (glass containers must not be used), and then dispense the diluted sample into the assay wells. Note that diluted samples tend to become more viscous; therefore, pipetting should be performed with care.
The standard dilution is 1:100; however, samples with high Apo B-48 levels may require dilutions of 1:200 or higher. When diluting samples, mix thoroughly while avoiding foam formation (1,800–2,000 rpm for 10 seconds x3).
- Are there any substances that may interfere with the assay?
- Fujifilm Wako have confirmed that no interference occurs at the concentrations shown in the table below. Do not use samples exhibiting marked hemolysis or those with a high lipid content.
Hemolysis No influence up to 160 mg/dL Chyle No influence up to 1,000 FTU Bilirubin-F No influence up to 10 mg/dL Bilirubin-C No influence up to 10 mg/dL
About kit usage
- What instruments, equipment and reagents are required for the assay using this kit?
- The instruments, equipment and reagents required for the use of this kit are listed below.
- Deionized water (or Distilled water)
- Test tubes for preparation of standard solution series
- Test tubes for sample storage (made of polypropylene (PP) or polyethylene (PE); glass containers are not suitable)
- Glassware for dilution of Wash Solution (10x) (a graduated cylinder, a bottle)
- Pipettes (disposable tip type, One should be able to deliver 50 µL precisely, and another for 200µL and 400µL)
- Syringe-type repeating dispenser (ex. Eppendorf multipette plus)
- Paper towel to remove washing buffer remaining in wells
- A vortex-type mixer
- A shaker for 96 well-plate (600-1,200 rpm)
- An automatic washer for 96 well-plate (if available), or a wash bottle with a jet nozzle.
- A 96 well-plate reader (450 nm±10 nm, 620 nm: 600 nm-650 nm)
- Software for data analysis
- How should each reagent be stored, and what are the shelf life guidelines?
Antibody-coated plate
For unused antibody-coated strips (kept refrigerated and with the seal intact), return them to the supplied zip-seal pouch and store at 2-10°C.Human Apo B-48 Standard
As a rule, prepare immediately before use. Once reconstituted, keep the stock solution refrigerated and use within 24 hours. If storage is unavoidable, freeze immediately after reconstitution at -35°C or below.Buffer and TMB Solution
When using only part of a solution, transfer slightly more than the required volume to a separate container. Immediately close the original container with the remaining solution without allowing it to reach room temperature, and store at 2-10°C.Biotin-conjugated Antibody Solution / Peroxidase-conjugated Streptavidin Solution
When using only a portion of the kit, remove the vial from the refrigerator only at the time of dilution. After dispensing the required amount, immediately close the cap tightly without allowing the remaining stock solution to reach room temperature, and store at 2-10°C. Discard any remaining diluted solution after use.Stop Solution
If the remaining solution is to be stored after use, close the cap tightly and store at 2-10°C.Wash Buffer (10x)
If storing 10x Wash Buffer, close the cap tightly and store at 2-10°C. Discard any remaining diluted Wash Buffer after use.
- How should the standard be prepared?
- To reconstitute the Human Apo B-48 Standard (lyophilized), add 400 µL of deionized water (or distilled water) equilibrated to room temperature to the vial, then let it stand at room temperature for 10 minutes. Mix thoroughly while avoiding foam formation (1,800–2,000 rpm for 10 seconds x3), and ensure that the lyophilized material is completely dissolved and the solution is clear.
[Precautions when mixing using a vortex mixer]
Place the vial on the vortex mixer before turning it on, then switch the mixer on to begin mixing. Do not place the vial on an already rotating mixer, as this may cause foaming.
The reconstituted stock solution is highly viscous. Before preparing the serial dilutions, ensure you are accustomed to pipetting highly viscous solutions. Accurate pipetting of the first 200 µL in the series is particularly critical.
When preparing each standard during the serial two-fold dilution, thorough mixing at every step is required (1,800-2,000 rpm for 10 seconds x3). Avoid foaming during mixing, as it can reduce assay reproducibility.
- Does Fujifilm Wako supply serum controls for use in the assay?
- Yes. We offer control serum for quality control purposes.
- Can I divide plates?
- Yes. Separate the strips by cutting along the boundaries between strips in the transparent seal on the plate using a utility knife or similar tool. Store any unused strips in the refrigerator with the seal left in place.
Troubleshooting
- What could be the possible causes when all wells show weak reactions?
- When all wells show weak reactions, the following causes may be considered:
- Adding the standard or samples was omitted
- Adding reagents related to color development was omitted
- Incorrect reagent selection or improper dilution of color-development reagents
- Contamination with enzyme inhibitors
- Effects of improper kit storage temperature (e.g., freezing)
- Excessive washing of the plate
- Low temperature of the TMB Solution
- What could cause the OD value of the blank to be higher than the OD of the lowest standard (2.5 ng/mL)?
- If the OD value of the blank exceeds the OD of the lowest standard (2.5 ng/mL), incomplete or inadequate washing is often the cause. Increase the number of wash cycles after the reaction with the peroxidase-conjugated streptavidin solution from four to five to eight times at the same flow rate.
- What could cause a high coefficient of variation (CV)?
- A high CV may result from one or more of the following:
- Incomplete or inadequate washing
- Insufficient mixing of the standard, control serum, or samples (ensure that frozen samples are thoroughly mixed after thawing )
- Inconsistent pipetting technique
Overview / Applications
Property
Manufacturer Information
Alias
- AKHB48(previous code)
637-10641(previous code)
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.