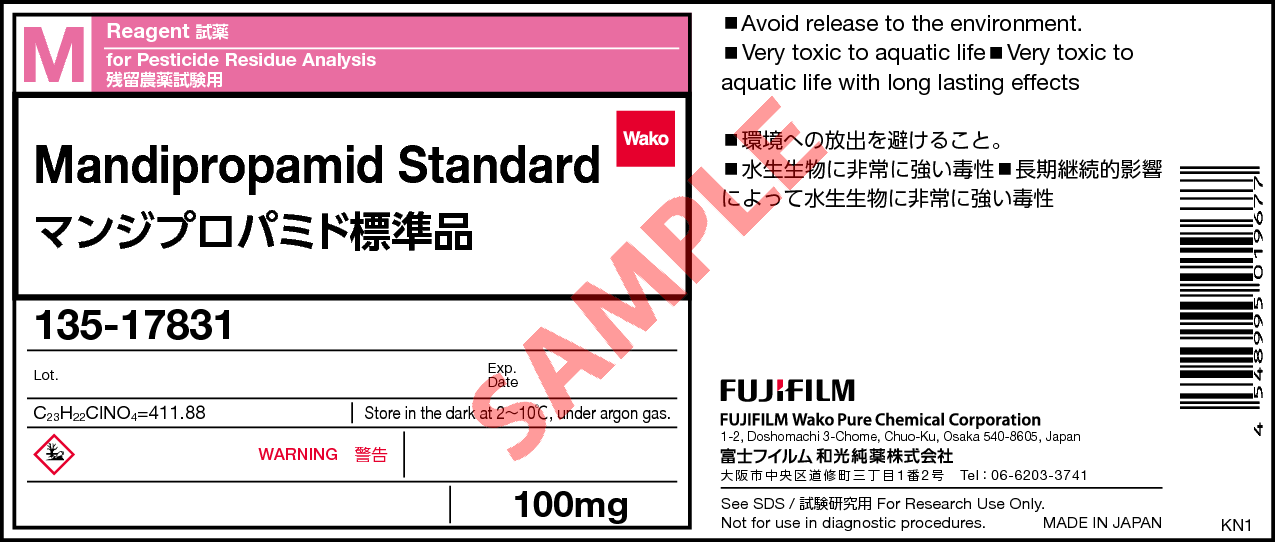
Mandipropamid Standard
- for Pesticide Residue Analysis
- Specification Assay :
- 98.0+% (qNMR), 98.0+% (HPLC)
- Manufacturer :
- FUJIFILM Wako Pure Chemical Corporation
- Storage Condition :
- Keep at 2-10 degrees C.
- CAS RN® :
- 374726-62-2
- Molecular Formula :
- C23H22ClNO4
- Molecular Weight :
- 411.88
- GHS :
-
- Structural Formula
- Label
- Packing
- SDS
|
Comparison
|
Product Number
|
Package Size
|
Price
|
Availability
|
Certificate of Analysis
|
Purchase |
|---|---|---|---|---|---|---|
|
|
|
100mg
|
|
In stock in Japan |
※Check availability in the US with the distributor.
Document
Overview / Applications
| Outline | This product is for research use only. Do not administer it to human.
|
|---|---|
| Precautions for Use | Packed on argon gas |
Property
| Appearance | White - slightly yellowish brown, crystalline powder - powder |
|---|
Manufacturer Information
Alias
- (RS)-2-(4-Chlorophenyl)-N-[3-methoxy-4-(prop-2-ynyloxy)phenethyl]-2-(prop-2-ynyloxy)acetamide
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.