GGsTop (TM)
- for Cellbiology
- Manufacturer :
- FUJIFILM Wako Pure Chemical Corporation
- Storage Condition :
- Keep at -20 degrees C.
- CAS RN® :
- 926281-37-0
- Molecular Formula :
- C13H18NO7P
- Molecular Weight :
- 331.26
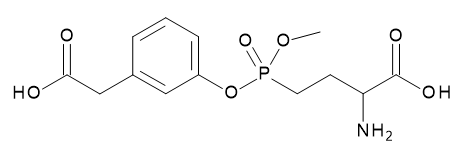
- Structural Formula
- Label
- Packing
- SDS
|
Comparison
|
Product Number
|
Package Size
|
Price
|
Availability
|
Certificate of Analysis
|
Purchase |
|---|---|---|---|---|---|---|
|
|
|
10mg
|
|
In stock in Japan |
||
|
|
|
100mg
|
|
In stock in Japan |
※Check availability in the US with the distributor.
Document
Overview
GGT, which is also known as γ-GT (γ-glutamyltransferase) or γ-GTP (γ-glutamyl transpeptidase) is a cell membrane-bound enzyme that hydrolyzes the γ-glutamyl bond between the Glu and Cys-Gly of glutathione (γ-Glu-Cys-Gly). GGT is a key enzyme in glutathione metabolism, as well as affecting intracellular glutathione levels, and it is thought to be involved in many diseases such as cardiovascular disease, type 2 diabetes mellitus, and the resistance of cancer cells to chemotherapeutic agents.
GGsTop® is a highly selective GGT inhibitor. Acivicin (AT-125), which has been widely used as a GGT inhibitor, inhibits glutamine amidotransferase (GA family) in addition to GGT, while GGsTop® shows high specificity for GGT without showing inhibitory activity against the GA family. It has also been reported to promote the production of collagen and elastin, and is being applied in the field of cosmetics.
Features
- Highly specific to GGT
- High inhibitory activity for human GGT
- Low toxicity
- Chemically stable
Inhibitory activity against human and E. coli GGTs
| kon (M-1s-1) | ||
|---|---|---|
| Human | E. coli | |
| GGsTop® | 51 | 170 |
| Acivicin | 0.40 | 4,200 |
GGsTop® shows approximately 100 times greater inhibitory activity for human GGT, when compared to acivicin.
kon: second order rate constant for enzyme inhibition (inactivation)
Inhibitory activity against asparagine synthetase
| Inhibitory concentration | |
|---|---|
| GGsTop® | > 10mmol/L (No inhibitory activity) |
| Acivicin | 100μmol/L (90% or greater inactivation after 2 hours) |
GGsTop® was confirmed to be a highly specific inhibitor of GGT as it did not inhibit E. coli asparagine synthetase even at a concentration approximately 100 times higher than that of acivicin.
Dissolution stability
Stable for at least 1 month at room temperature in neutral water or acidic aqueous solution (e.g. :0.1% TFA solution and 0.1 N HCl solution). Avoid dissolving this product in alkaline solution (pH 9 or higher) due to the consequent instability.
Usage
Dissolve this product in distilled water to prepare a 10 mmol/L stock solution, divide into small aliquots, and store at -20°C. Avoid repeated freeze-thaw cycling of the stock solution.
Storage conditions
Store at -20°C.
As this product is highly hygroscopic, immediately stopper or seal the container after use. If moisture is absorbed, it can be used without problems after drying in a desiccator under vacuum, using a desiccant such as diphosphorus pentoxide.
References
- Han, L. et al. : Biochemistry, 46, 1432 (2007). Design, Synthesis, and Evaluation of γ-Phosphono Diester Analogues of Glutamate as Highly Potent Inhibitors and Active Site Probes of γ-Glutamyl Transpeptidase
- Hiratake J.:和光純薬時報, 76(3), 2 (2008). (Japanese)
- Yamamoto, S. et al. : J. Pharmacol. Exp. Ther., 339, 945 (2011). Preventive Effect of GGsTop, a Novel and Selective γ-Glutamyl Transpeptidase Inhibitor, on Ischemia/Reperfusion-Induced Renal Injury in Rats
- Brady, M. J. et al. : Current Enzyme Inhibition, 7, 71 (2011). Inhibiting Glutathione Metabolism in Lung Lining Fluid as a Strategy to Augment Antioxidant Defense
- Yuasa, A., et al. : Journal of Japanese Cosmetic Science Society, 36(2), 93 (2012). A γ-Glutamyl Transpeptidase (GGT) Inhibitor Enhances Collagen and Elastin Synthesis (Japanese)
- Kubota, R. et al. : Br. J. Pahrmacol., 177(22), 5195 (2020). Inhibition of γ-glutamyltransferase ameliorates ischaemia-reoxygenation tissue damage in rats with hepatic steatosis
- Ichikawa, S et al. : Int. J. Oral-Med. Sci., 18(3)(4), 183 (2020). A GGT Inhibitor Suppresses IL-6 and IL-8 Expressions Enhanced by LPS in Gingival Fibroblasts
- Jiang, Y. et al. : Mol. Med. Rep., 13(5), 3813 (2016). GGsTOP increases migration of human periodontal ligament cells in vitro via reactive oxygen species pathway
- Terzyan, S. S. et al. : J. Biol. Chem., 290(28), 17576 (2015). Human γ-Glutamyl Transpeptidase 1: STRUCTURES OF THE FREE ENZYME, INHIBITOR-BOUND TETRAHEDRAL TRANSITION STATES, AND GLUTAMATE-BOUND ENZYME REVEAL NOVEL MOVEMENT WITHIN THE ACTIVE SITE DURING CATALYSIS
- Sakuma, K., et al. : Journal of the Japanese Society for Disability and Oral Health, 41(1), 1 (2020). The effect of γ-glutamyl transpeptidase inhibitor on wound healing of oral mucosa (Japanese)
- 11.Baumann, T. et al. : Exp. Dermatol., 23(4), 247 (2014). Glutathione-conjugated sulfanylalkanols are substrates for ABCC11 and γ-glutamyl transferase 1: a potential new pathway for the formation of odorant precursors in the apocrine sweat gland
- Mizushima, T. et al. : BMC Cancer, 16, 411 (2016). Fluorescent imaging of superficial head and neck squamous cell carcinoma using a γ-glutamyltranspeptidase-activated targeting agent: a pilot study
- Tobe, T. et al. : J. Toxicol. Sci., 42(1), 85 (2017). Selenium uptake through cystine transporter mediated by glutathione conjugation
- Kubo, H. et al. : Sci. Rep., 8, 17781 (2018). Rapid detection of metastatic lymph nodes of colorectal cancer with a gamma-glutamyl transpeptidase-activatable fluorescence probe
- Akashi, T. et al. : Sci. Rep., 9, 9467 (2019). A novel method for rapid detection of a Helicobacter pylori infection using a γ-glutamyltranspeptidase-activatable fluorescent probe
Overview / Applications
| Outline | This product is for research use only. Do not administerit to human. Highly Selective γ-Glutamyl transpeptidase (GGT) Inhibitor GGT, which is also known as GTP, GPT, γ-GTP, γ-GT, γ-GPT, γ-glutamyltransferase, γ-glutamyl transpeptidase or γ-glutamyl peptidyltransferase (EC 2.3.2.2) is a cell membrane-bound enzyme that hydrolyzes γ-glutamyl bond between Cys and Gly of glutathione (γ-Glu-Cys-Gly). GGT is involved in a number of biological events such as drug resistance and metastasis of cancer cells by detoxification of xenobiotics and reactive oxygen species through glutathione metabolism, and is also implicated in physiological disorders, such as Parkinson's disease, neurodegerative disease, Type II diabetes and cardiovascular diseases. GGsTopTM is a highly selective γ-Glutamyl Transpeptidase (GGT) inhibitor. While acivicin [AT-125], which has been widely used as a GGT inhibitor also inhibits asparagine synthetase (GA family); GGsTopTM does not inhibit the asparagine synthetase. Features
On acute toxicity test, there is no toxicity with intravenously-infused GGsTopTM (30 mg/kg). On the other hand, acivicin has sever toxicity to the CNS. The reconstituted neutral or acid aqueous solution such as 0.1% TFA solution and 0.1N HCl solution is stable for 1 month at room temperature. Avoid reconstituting GGsTopTM with alkaline solution (> pH 9) due to the instability. [References] |
|---|---|
| Precautions for Use | Packed on argon gas |
Property
| Appearance | White - slightly brown, crystals - powder or mass |
|---|
Manufacturer Information
Alias
- 2-Amino-4-{[3-(carboxymethyl)phenyl](methyl)phosphono}butanoic Acid
GGT Inhibitor
γ-GTP Inhibitor
γ-Glutamyltransferase Inhibitor
γ-Glutamyl Transpeptidase Inhibitor
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.