Sodium Bicarbonate [CertiPro:JP,Ph.Eur.,Endotoxin test]
- Japanese Pharmacopoeia
- Specification Assay :
- 99.0+% (NaHCO3)(Titrarion)(JP)
99.0 - 101.0% (NaHCO3)(Titration)(Ph.Eur.)
- Manufacturer :
- FUJIFILM Wako Pure Chemical Corporation
- Storage Condition :
- Keep at RT.
- CAS RN® :
- 144-55-8
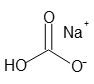
- Molecular Formula :
- NaHCO3
- Molecular Weight :
- 84.01
- Structural Formula
- Label
- Packing
|
Comparison
|
Product Number
|
Package Size
|
Price
|
Inventory
|
|
|---|---|---|---|---|---|
|
|
|
10kg
|
|
In stock in Japan |
|
|
|
|
500G
|
|
In stock in Japan |
Document
- Statement
- Product Specification Sheet
- Package Insert
-
- Spectral Data
-
*For details of Statement, see Click here "Statement"
Application
Overview / Applications
| Outline | This is a raw material for pharmaceuticals. It is a compliant product that added the European Pharmacopoeia (EP) test to the Japanese Pharmacopoeia (JP) standard. We have conducted endotoxin test and standardized it. When purchasing, a confirmation letter at the time of selling pharmaceuticals exclusively for manufacturing is necessary. |
|---|
Property
| Appearance | White, crystals or crystalline powder (JP) |
|---|---|
| pH | pH : 7.9 - 8.4 (JP) |
| Purity | Bacterial endotoxins : <10EU/g |
Manufacturer Information
Alias
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.