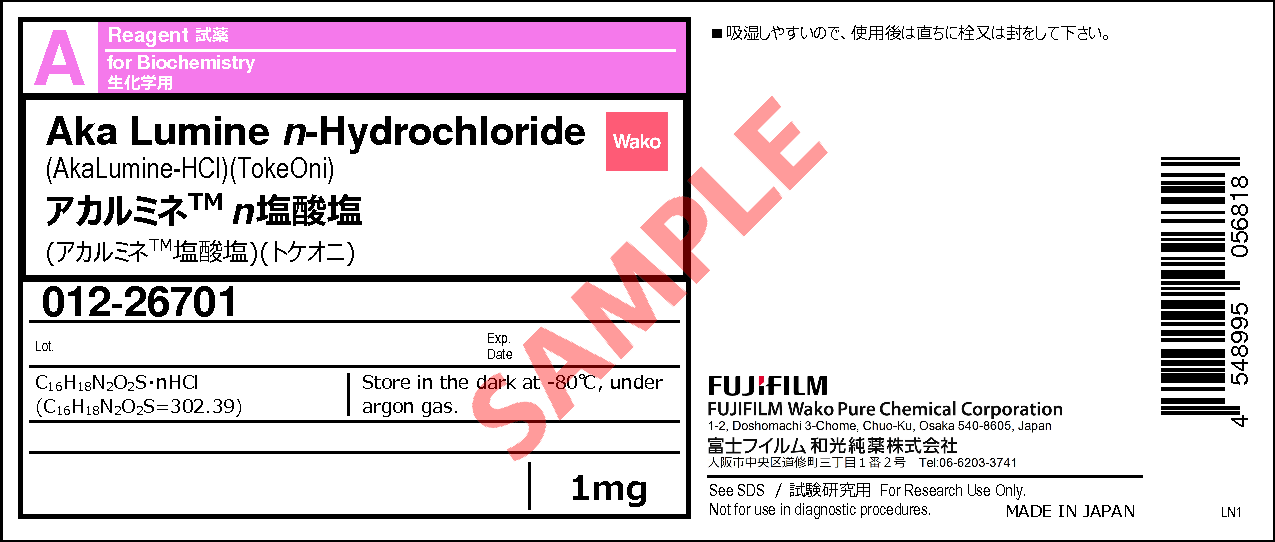
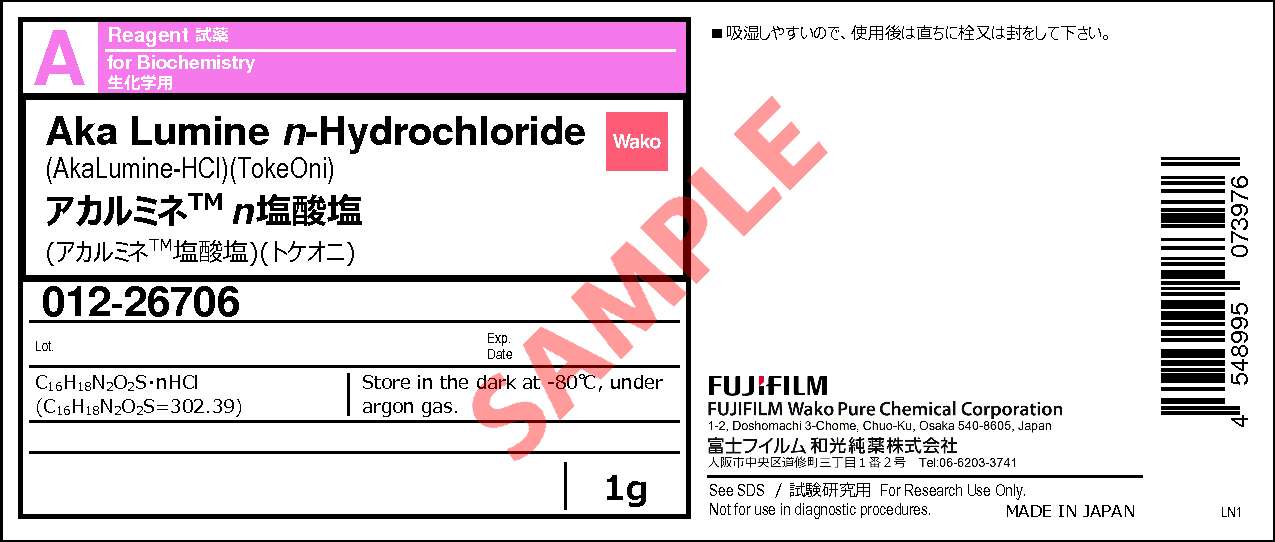
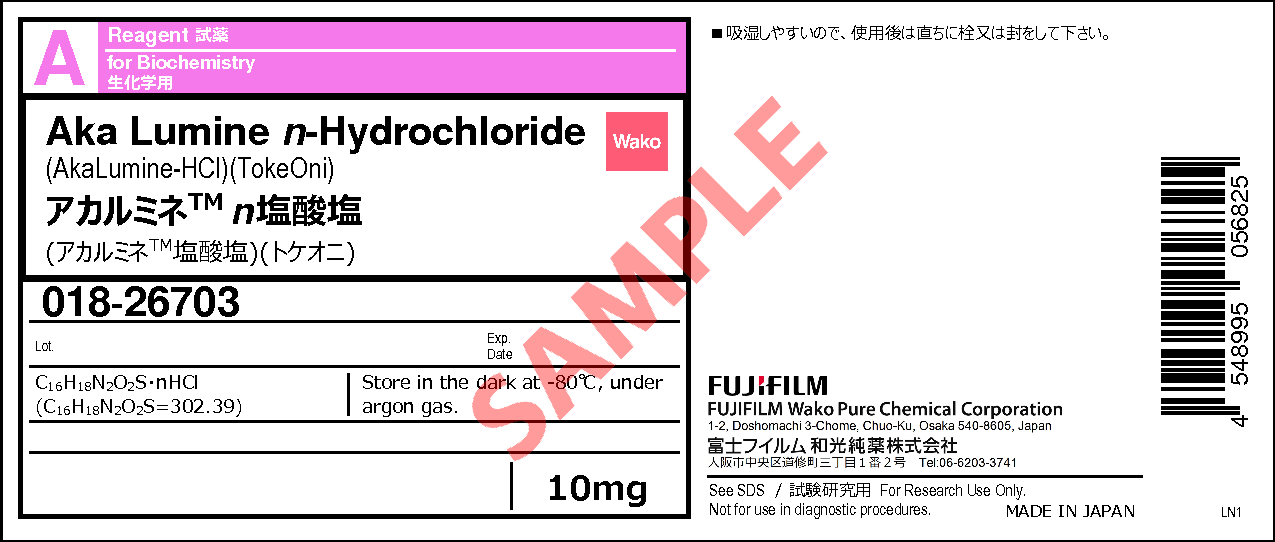
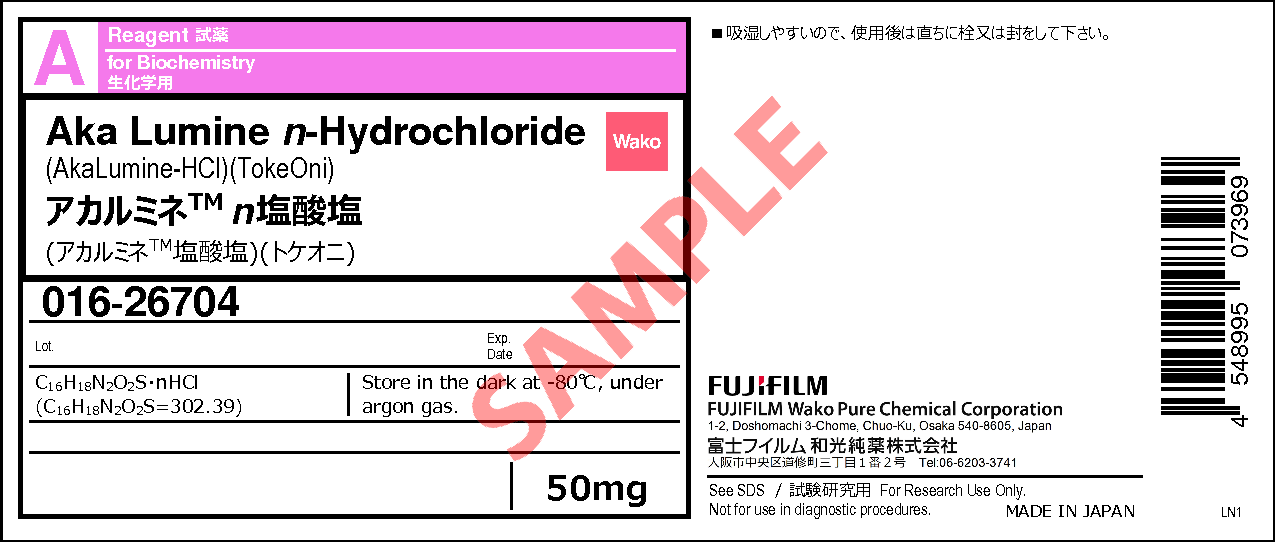
Aka Lumine n-Hydrochloride
- for Biochemistry
- Specification Assay :
- 80+% (HPLC)(D-form)
- Manufacturer :
- FUJIFILM Wako Pure Chemical Corporation
- Storage Condition :
- Keep at -80 degrees C.
- CAS RN® :
- 1176235-08-7(no salt)
- Molecular Formula :
- C16H18N2O2S・ nHCl
- Molecular Weight :
- (C16H18N2O2S=302.39)
- Structural Formula
- Label
- Packing
- SDS
|
Comparison
|
Product Number
|
Package Size
|
Price
|
Inventory
|
|
|---|---|---|---|---|---|
|
|
|
1mg
|
|
In stock in Japan |
|
|
|
|
1G
|
|
||
|
|
|
10mg
|
|
In stock in Japan |
|
|
|
|
50mg
|
|
In stock in Japan |
Document
Application
Overview / Applications
| Outline | This product is for research use only. Do not administer it to human. Akalumine-HCl is a luminescent substrate of luciferase used for in vivo bioluminescence imaging (in vivo BLI). This product has wavelength at near infrared region (670-680nm) that less susceptible to absorb by water and hemoglobin than luciferin. Therefore, it's possible to observe more deep inside the body. In vivo BLI is a noninvasive imaging method for using luciferase expressing cells. This method causes luminescence signals in an animal with luciferase expressing cells by administering luminescent substrate such as luciferin and Akalumine-HCl. The signals are detected by high sensitivity camera. The example of use is artificial bioluminescence system called as AkaBLI, developed by Dr. Atsushi Miyawaki and Dr. Satoshi Iwano etc. This method uses Akaluc as luciferase and Akalumine-HCl as luciferin. It's possible to detect deep tissues noninvasively at the single cell level and track cell activity over time in the same living animals. See the following website for more details. https://labchem-wako.fujifilm.com/us/category/00669.html |
|---|---|
| Precautions for Use | Packed on argon gas |
Property
| Appearance | Dark reddish purple - black, crystalline powder - powder or paste |
|---|
Manufacturer Information
Alias
- (AkaLumine-HCl) (TokeOni)
TokeOni
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.