Blood Stain/Semen Detection Reagents
Although detection of bloodstains is an important task in forensic science, sometimes it is difficult to distinguish bloodstains from other stains. As examples of tests for this purpose, luminol reaction and leucomalachite green reaction are used in a preliminary test to determine whether a stain is a bloodstain. Fujifilm Wako provides a set of reagents for the luminol reaction and the leucomalachite green reaction, both of which require little effort for reagent preparation.
The presence of prostatic acid phosphatase proves the presence of semen. We also provides SM reagents , which can detect the acid phosphatase.
More Information
Principle of Detection for Bloodstains
A preliminary bloodstain test is performed first to determine whether a stain is a bloodstain or not. Luminol reaction reagents, leucomalachite green reaction reagents, and the like are used in the preliminary bloodstain test.
Luminol
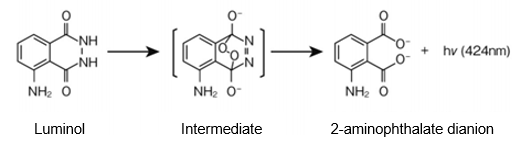
Luminol (5-amino-2,3-dihydro-1,4-phthalazinedione) is catalyzed by a variety of substances with peroxidase-like activity (iron, copper, cobalt, foliage, or hemoglobin) in alkaline aqueous solution to form a diazaquinone intermediate and then form a phthalate dianion in the excited state. As the reaction proceeds further, 2- aminophthalate dianion is formed and light is emitted when it returns to the ground state (Figure 1).
As hemoglobin contained in blood acts as a catalyst for the luminol reaction, bloodstains can be detected by a violet-blue luminescence. However, luminol may react with peroxidases other than blood, such as plant-derived peroxidases, and thus the specificity of luminol is said to be lower than that of leucomalachite green.
Leucomalachite Green
Although leucomalachite green is generally colorless, it is converted to blue-green malachite green when oxidized by a redox reaction catalyzed by hemoglobin in blood. This detection is based on color development and less sensitive than luminol, whereas it is said to have a high specificity for blood because it seldom reacts with plant-derived peroxidases. A possible explanation for this is that the hydrogen peroxide concentration in leucomalachite green reagents is too high for a peroxidase, resulting in the formation of an inactive reaction intermediate of peroxidase1).
Principle of Detection for Semen
To determine whether a stain is semen or not, a biochemical test is performed in addition to appearance, microscopic examination, and serologic test.
A large quantity of prostate-derived acid phosphatase is present in human semen. α-naphthylphosphoric acid is hydrolyzed into α-naphthol by acid phosphatase, and α-naphthol reacts with diazonium o-dianisidine, which turns purple. The SM reagents composed of α-naphthylphosphoric acid and diazonium o-dianisidine have high sensitivity and have been widely used as a semen detection method.
References
- 1) Ohmori, T and Hosoya, T.: Japanese journal of science and technology for identification, 7(2),155(2003)
Effect of Hydrogen Peroxide Concentration on a Coloring Reaction of Leucomalachite Green - Comparison of Hemoglobin with Peroxidase - (Japanese)
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



