Reference Material (SI Traceable)
Grade for our reference material
Based on international standards such as ISO/IEC 17025, National Institute of Technology and Evaluation International Accreditation Japan (NITE IAJapan) which accredits testing and calibration laboratories operates multiple accreditation systems. The accreditation system for reference material (RM) includes Japan Calibration Service System (JCSS) which is a registration/accreditation program based on the Measurement Law of Japan, and Accreditation System of National Institute of Technology and Evaluation (ASNITE) which is an accreditation program that complements areas that cannot be covered by other accreditation programs based on domestic law.
RMs Fujifilm Wako supplies have the following characteristics.
- Ensured metrological traceability to metrological references (JCSS: National standard of Japan, ASNITE: National standard of Japan and other countries)
- CRM (Certified Reference Material) *1
- A certificate with ASNITE RM Producer Accreditation Symbol is attached to ASNITE CRM, and a certificate with JCSS Accreditation Symbol is attached to JCSS CRM. Certificates with these accreditation symbols indicate that they meet all of the requirements for CRMs in ISO 17034.*2
ISO 17034 was established by Asia-Pacific Accreditation Cooperation (APAC) to standardize the method of evaluating the ability of RM producers in each country. The certificates with the ASNITE accreditation symbol are accepted not only domestically but also internationally by APAC Mutual Recognition Agreement (MRA) *3
The table below shows the relationship between each specification of RM and the accreditation system.
| Specification | (1) JCSS | (2) Mixture Standard Solution for Amino Acid Automated Analysis |
(3) Standard Solution for ICP Analysis | (4) Reference Material for Pesticide Residue Analysis | (5) Standard Solution for Volumetric Analysis (for the Japanese Pharmacopeia General Tests)*2 |
(6) TraceSure® | (7) TRM | Our Analytical Standard | |
|---|---|---|---|---|---|---|---|---|---|
| Item | Foul smell, VOC, Element, pH |
Amino acid | Element | Pesticide | Volumetric analysis | Volumetric analysis | Pesticide, qNMR analysis | Pesticide, Amino acid |
Others |
| Accreditation system |
JCSS | ASNITE | - | ||||||
| Accreditation system based on the Measurement Law |
Accreditation programs operated by IAJapan to complement private areas not covered sufficiently by other accreditation programs. | - | |||||||
| Accreditation government |
IAJapan | - | |||||||
| Accreditation symbol |
- | ||||||||
| Metrological reference |
CERI specific RM |
- | NIST SRM, etc. | TraceSure® | NMIJ CRM | NMIJ / CERI calibration | - | ||
| Traceability | SI traceable | - | |||||||
| MRA compatible | ✔ | - | |||||||
*1 Fujifilm Wako's JCSS meets all requirements of ISO/IEC 17025 [2017(JIS Q 17025)] and ISO 17034 [2016(JIS Q 17034)], which are qualified to be a CRM.
*2 There is no certificate attached to the standard solution for volumetric analysis (for the Japanese Pharmacopeia General Tests).
*3 Chemical Evaluation and Research Institute, Japan's HP
Product Line-up
More Information
JCSS
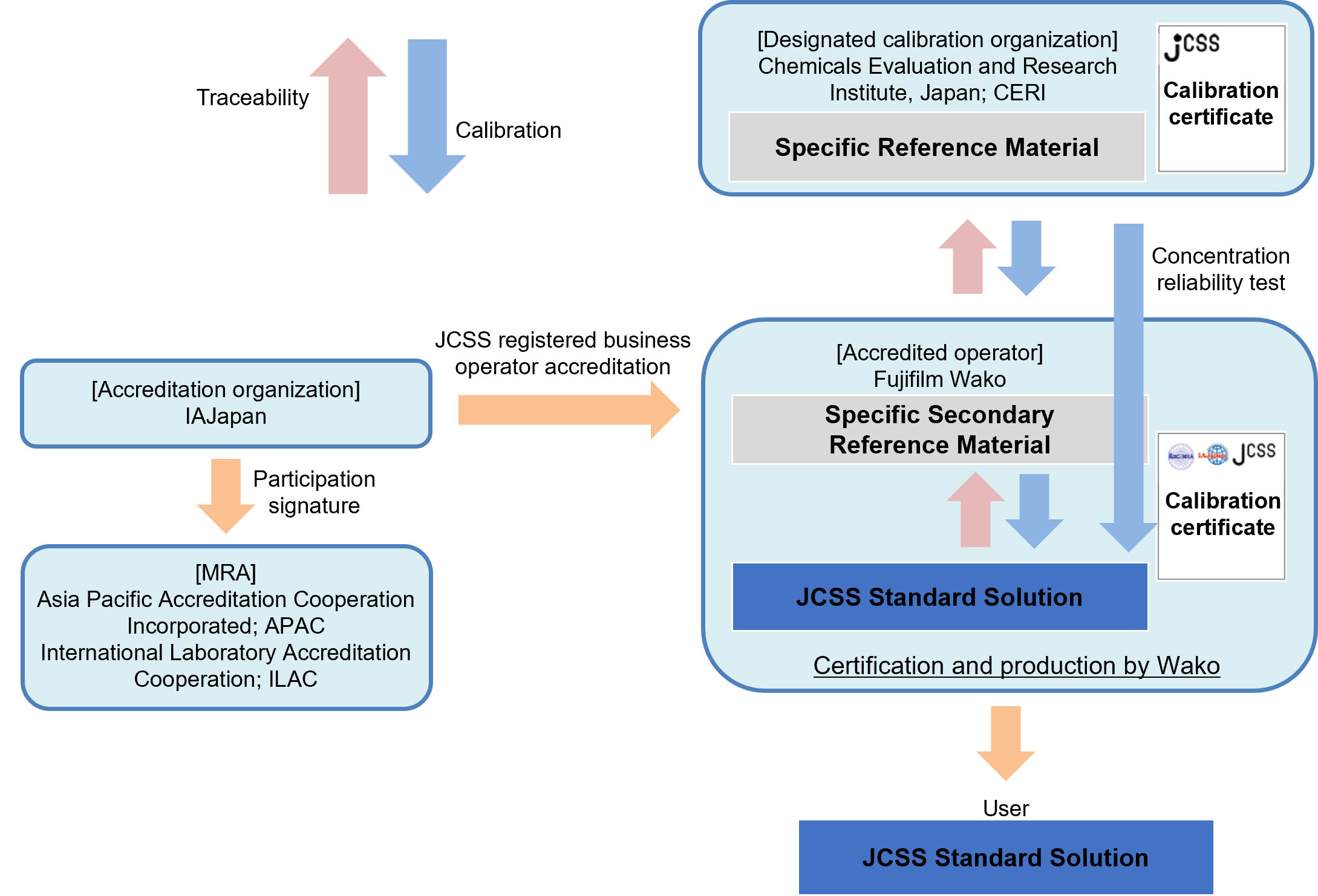
JCSS (Japan Calibration Service System) is a traceability system based on the Measurement Law of Japan. It is developed from time to time and used as a reference material for various official methods in Japan. JCSS reference materials are SI traceable CRM (certified reference materials) that ensure traceability to specific reference materials, manufactured by CERI (Chemicals Evaluation and Research Institute, Japan), a designated calibration institute under the Measurement Law of Japan. JCSS standard solutions are accompanied by a calibration certificate bearing the IAJapan and MRA (Mutual Recognition Agreement) symbols, and the concentrations on the calibration certificate are accepted internationally through the MRA of ILAC/APAC. Fujifilm Wako provides JCSS reference materials such as inorganic standard solutions (ion/metal standard solutions), organic standard solutions, and pH standard solutions.
Traceability of JCSS
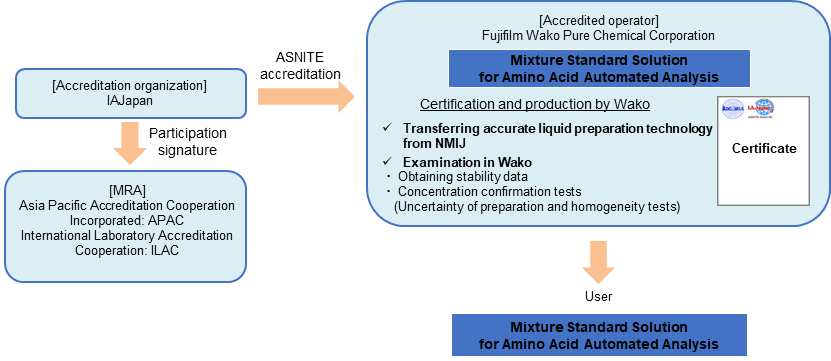
Mixture Standard Solution for Amino Acid Automated Analysis
In 2019, Fujifilm Wako accredited the “Mixture Standard Solution for Amino Acids Producer” for the first time in Japan based on ASNITE accreditation, and started the production of amino acids CRM that can be “yardstick” for amino acids measurement.
Amino acids are components that make up proteins, and are important nutritional components contained in various foods such as meat and grains. In the clinical field, technological development is progressing to screen future risks of cancer, diabetes, and stroke by measuring amino acids in blood. There are growing needs for more accurate evaluation of amino acids contents and composition in samples.
This product uses National Metrology Institute of Japan (NMIJ) CRM or our Traceable Reference Materials (SI traceable RM, TRM) as raw materials. Fujifilm Wako has established a RM production system based on ISO 17034 by transferring accurate liquid preparation technology from NMIJ, obtaining stability data, and examining concentration at Fujifilm Wako. The use of amino acids mixture standard solutions (CRM) makes it possible to assure more reliable amino acids analysis.
Traceability of "Mixture Standard Solution for Amino Acid Automated Analysis"
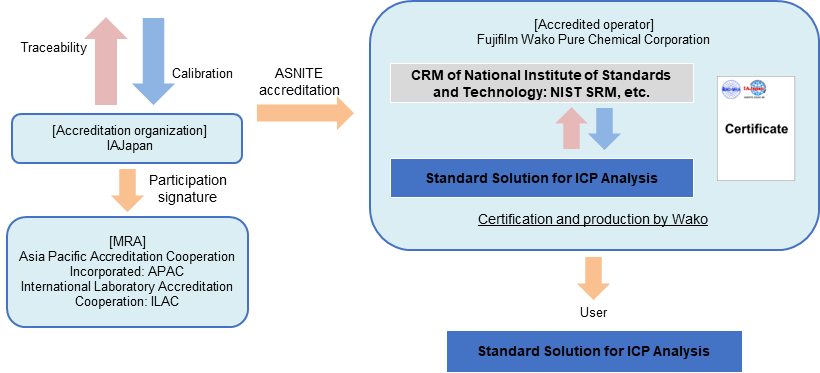
Standard Solution for ICP Analysis
From March 2023, Fujifilm Wako has become the first company in Japan to obtain comprehensive accreditation (flexible scope of accreditation) as an inorganic RM manufacturer based on ASNITE accreditation. "Flexible scope of accreditation as an inorganic RM manufacturer" is a system that allows element standard solution to be produced as CRM without obtaining individual accreditation, if the element standard solution concentration can be valued using a method accredited by an accreditation body. Fujifilm Wako will quickly supply CRM using flexible scope of accreditation.
The element standard solution for ICP analysis (CRM) series is a CRM whose concentration value has been valued using a method, which has received flexible scope of accreditation from IAJapan, and a CRM of National Institute of Standards and Technology (NIST SRM), etc.
Fujifilm Wako will sequentially renew ICP analysis grade of elemental standard solutions to SI traceable CRM.
Traceability of "Standard Solution for ICP Analysis (CRM)"
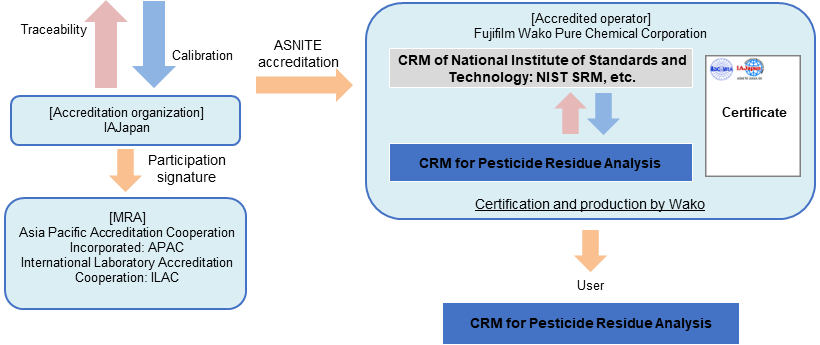
Reference Material for Pesticide Residue Analysis
From March 2023, Fujifilm Wako has become the first company in Japan to obtain comprehensive accreditation (flexible scope of accreditation) as an organic RM manufacturer based on ASNITE accreditation. "Flexible scope of accreditation as an organic RM manufacturer" is a system that allows analytical standard to be produced as CRM without obtaining individual accreditation, if analytical standard can be valued using a method accredited by an accreditation body. Fujifilm Wako will quickly supply CRM using flexible scope of accreditation.
The RM for Pesticide Residue Analysis series is a CRM whose purity value has been valued using a method, which has received flexible scope of accreditation from IAJapan, and a CRM of NIST SRM, etc.
Fujifilm Wako will sequentially renew “for Pesticide Residue analysis” grade of analytical standards to SI traceable CRM.
Our company has established procedures for developing and producing Certified Reference Materials (CRMs) using the hydrogen nucleus-based quantitative NMR method (1H qNMR method) and has obtained ISO 17034 accreditation (Accreditation for Reference Material Producers). We plan to leverage this method to flexibly develop CRM products going forward. A scientific article co-authored with USP regarding this research has been published.
Please see here for details.
Traceability of "Reference Material for Pesticide Residue Analysis (CRM)"
Standard Solution for Volumetric Analysis (for the Japanese Pharmacopeia General Tests)
The standard solution for volumetric analysis [for the Japanese Pharmacopoeia (JP) General Tests] series are CRMs that have been prepared and standardized in accordance with JP and can be used for the tests listed in JP.
Concentration value of this series is valued using the TraceSure® produced by Fujifilm Wako as the primary RM, and this TraceSure® series is valued using NMIJ CRM as the primary RM (refer to TraceSure®) .
Since the TraceSure® series is a CRM whose purity values are traceable to SI through the NMIJ CRM, the certified values (concentration) of this series are traceable to SI through TraceSure®.
Traceability of "Standard Solution for Volumetric Analysis (for the Japanese Pharmacopeia General Tests)"
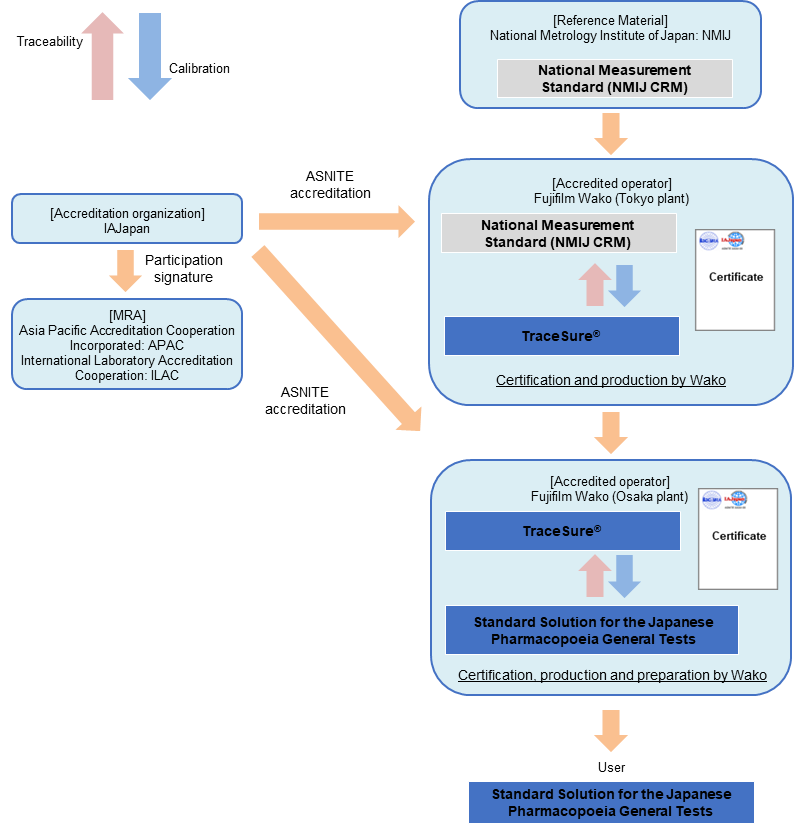
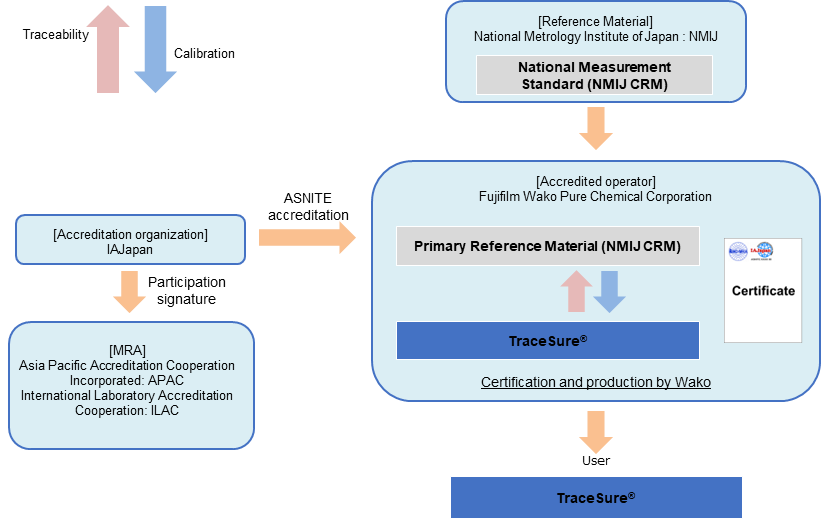
TraceSure®
Traceability of TraceSure® / Volumetric Analysis
TraceSure® for volumetric analysis is valued using the NMIJ CRM, which is valued by NMIJ using SI traceable measurement methods, and the purity value is determined by adding the uncertainty obtained from the homogeneity and stability evaluations by Fujifilm Wako. Purity values of TraceSure® are SI traceable via the NMIJ CRM.
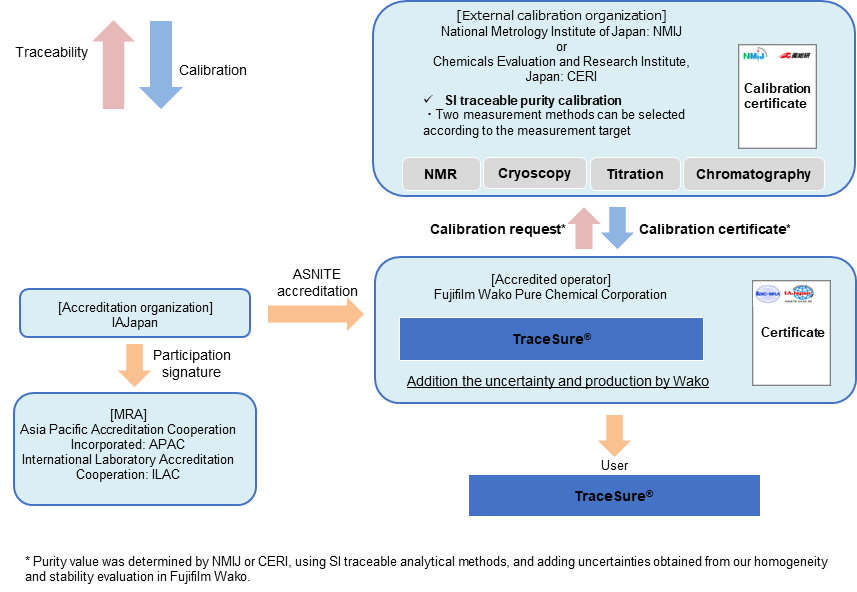
Traceability of TraceSure® / Pesticide Residue and qNMR Analysis
The TraceSure® "for pesticide residue analysis" and "for qNMR analysis" are a series of CRMs which are valued by NMIJ or CERI and whose purity values are determined by adding uncertainties obtained from homogeneity and stability evaluation in Fujifilm Wako. The purity values of these CRMs are traceable to SI through the analytical values of NMIJ or CERI.
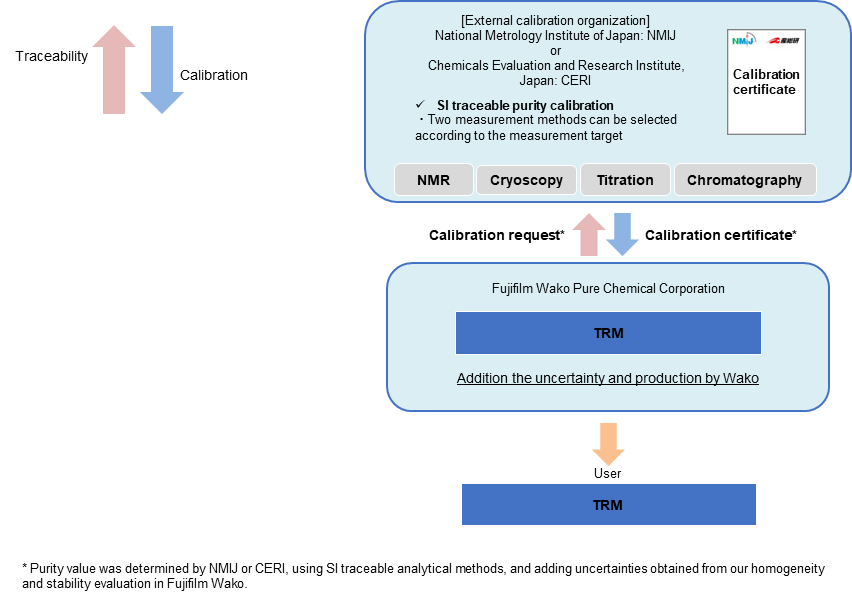
TRM (Traceable Reference Material)
The TRM is a series of RMs which are valued by NMIJ or CERI and whose purity values are determined by adding uncertainties obtained from homogeneity and stability evaluation in Fujifilm Wako. The purity values of these RMs are traceable to SI through the analytical values of NMIJ or CERI.*
*The TRM is not accredited as a producer of RMs under the ASNITE accreditation program (this series is non-CRM).
Traceability of TRM
Product Line-up
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



