In Silico Toxicity Prediction System - Free Trial
Fujifilm's In Silico Toxicity Prediction System is a Software as a Service (SaaS) type web application designed to efficiently predict mutagenicity and skin sensitization properties of chemical compounds with high accuracy and speed. Leveraging statistical models based on Fujifilm's proprietary data, the platform enables users to input chemical structures to evaluate key properties critical for safety screening, even for structurally diverse or novel compounds. Unlike many conventional QSAR tools that rely solely on machine learning or limited training sets, this system provides robust predictive accuracy and precision with in silico predictions of metabolic reactions and chemical reactivity, offering robust predictive power and accuracy for diverse chemical spaces. Its cloud-based design and intuitive UI ensures rapid calculations without the need for local installations, making it a highly valuable resource for both early-stage screening and regulatory compliance workflows.
In Silico Toxicity Prediction System: Privacy Policy & Terms of Use
Software Features
Rule-based model
- Equipped with a metabolic reaction simulator, it reproduces the metabolic reactions of chemicals, reducing the risk of false positives.
- For each metabolite generated by the metabolic reaction simulator, the mutagenicity is predicted using the rule-based model.
- If any metabolite has a positive structure, the compound is judged as positive.
Statistical-based model
- High-quality test data accumulated at Fujifilm is used for training.
- In addition to pharmaceuticals, a wide variety of structural data for applications like dyes, liquid crystals, and dispersed materials are also suitable, and accurate prediction of new compounds can be expected.
User Interface
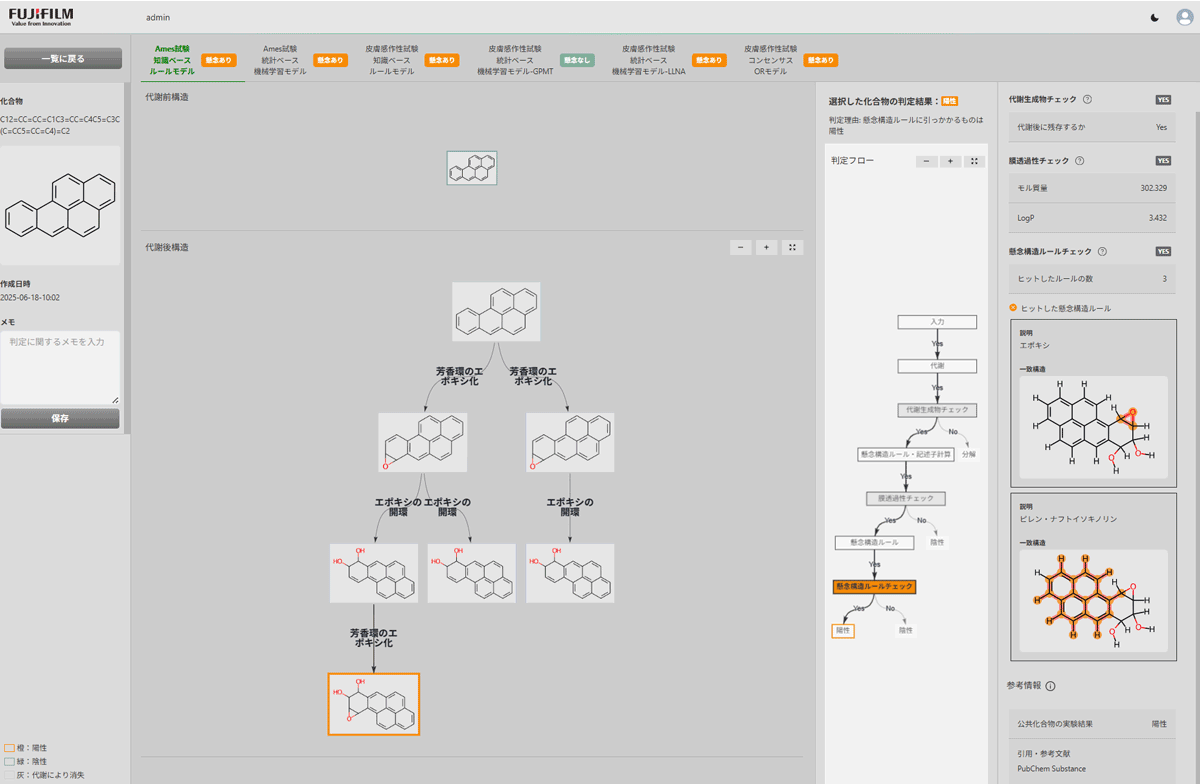
Confidentiality of information
- Compound data (structural formula) is handled only within the cloud system.
- To protect confidentiality, the system is isolated from third parties, including Fujifilm Corporation, the software developer.
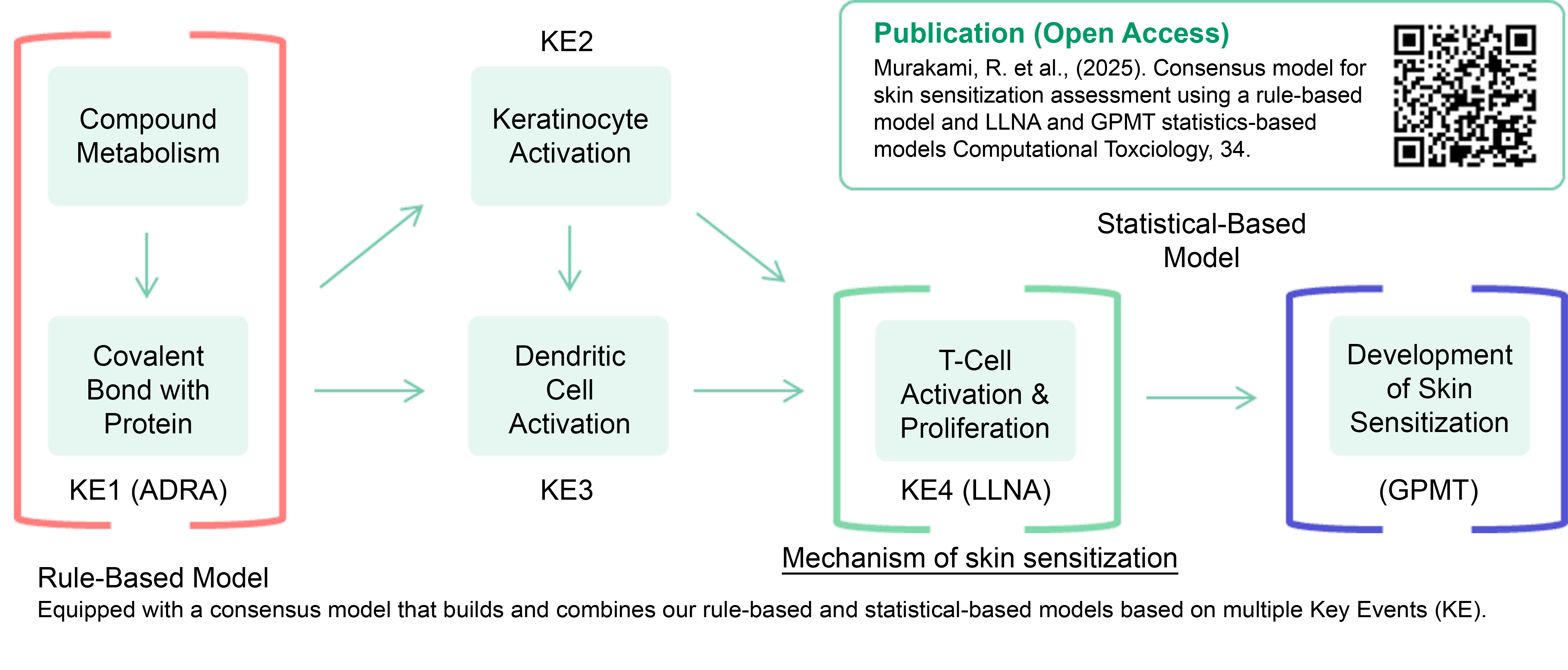
Two Types of QSAR Models: Rule-Based and Statistical-Based
The ICH-M7 guidelines recommend the use of two complementary QSAR models (knowledge-based and statistical).
FUJIFILM Corporation has developed an unprecedented QSAR software that combines two QSAR models: a rule-based model that incorporates a proprietary metabolic reaction simulator and a statistical-based model that incorporates various compound information cultivated through product development to date.
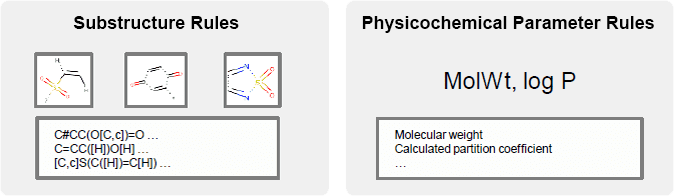
Rule-based model
Common to all positive chemicals
- Substructure (toxicity-causing structures)
- Rule-based physicochemical parameters
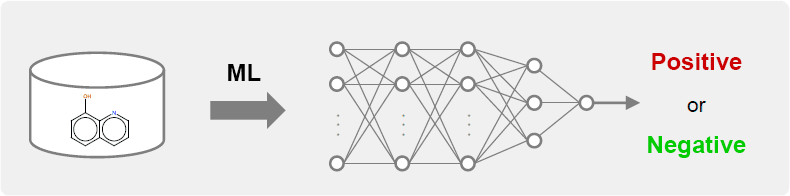
Statistical-based model (AI)
By machine learning,
- Estimated values of physical properties (descriptors)
- Models are trained based on features such as the presence or absence of specific structures (fingerprints)
*Presented at the 50th Annual Meeting of the Japanese Society of Toxicology (June 2023)
「Design and development of compound safety prediction system including AMES prediction considering metabolism」
Rule-Based Model: The Importance of Considering Metabolic Reactions
By taking metabolic reactions into account, “false negatives,” which imply an incorrectly predicted negative result for a substance that is actually positive, can be reduced.

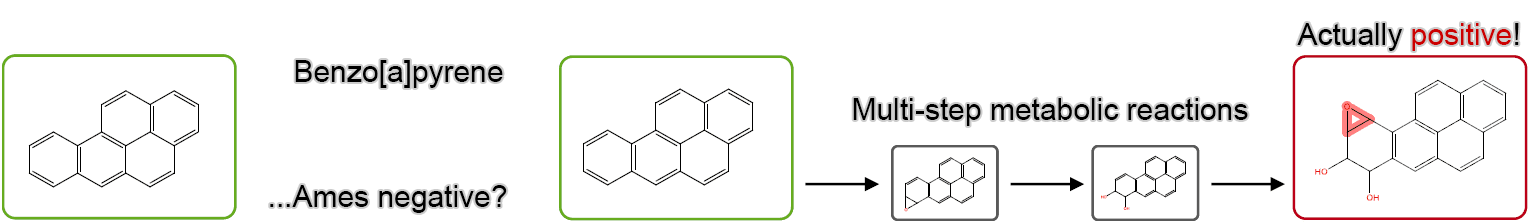
Rule-Based Model: Prediction Example
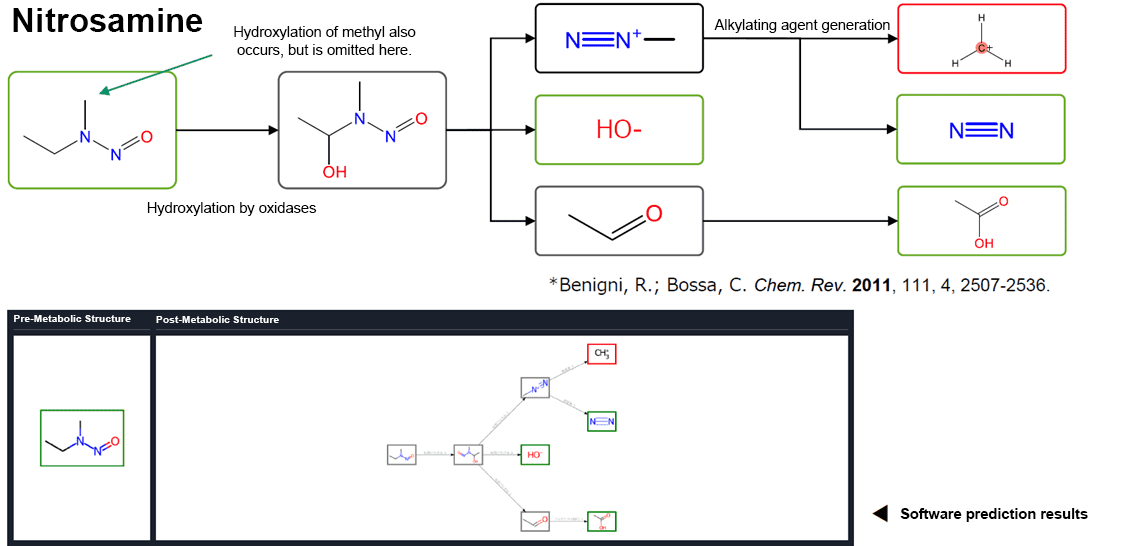
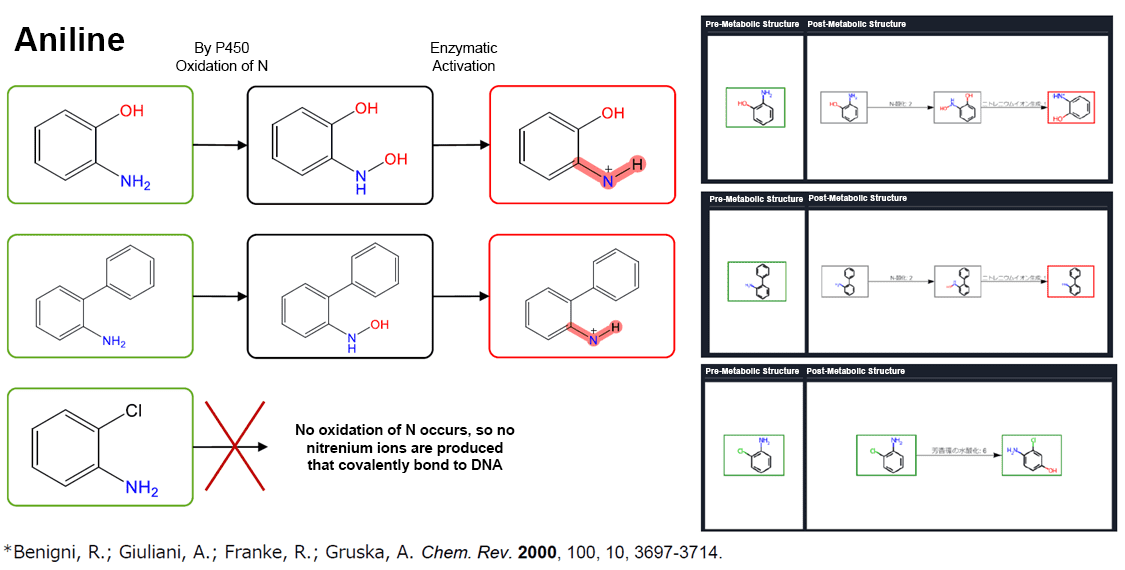
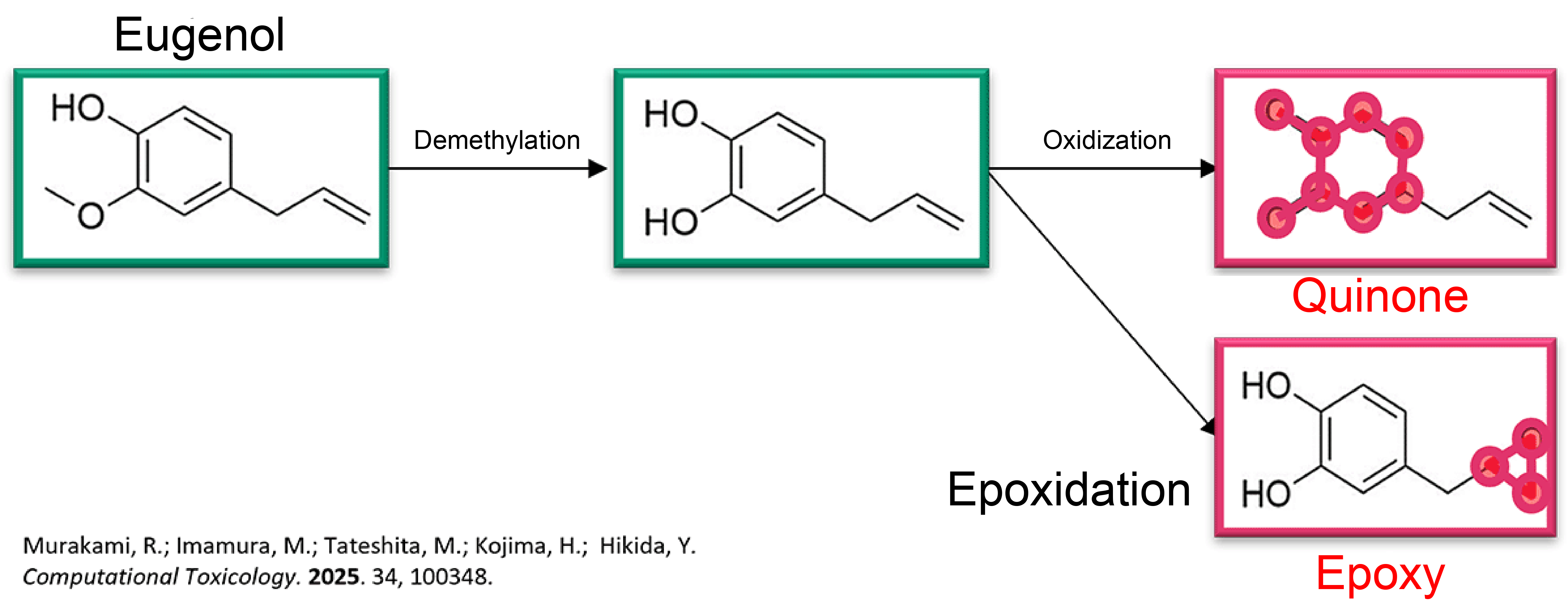
Rule-Based Model: Considering Metabolic Reactions — Evaluation of Prediction Performance
Comparison of prediction performance with and without considering metabolic reactions
Example 1: Ames
•Validated using about n=300 public data (Annual Meeting of the Japanese Society for Environmental Mutagenomics, 2023)
| Indicators | When S9 metabolism is not taken into account | When S9 metabolism is taken into account |
|---|---|---|
| Positive substances are judged as positive | 64% | 77% |
| Negative substance is judged as negative | 88% | 85% |
Example 2: Skin Sensitization Test
•Validated using about n=200 public data (2025 Annual Meeting of the Japanese Society of Toxicology)
| Indicators | When S9 Metabolism is not taken into account | When S9 metabolism is taken into account |
|---|---|---|
| Positive substances are judged as positive | 76% | 90% |
| Negative substance is judged as negative | 97% | 88% |
Statistical-Based Model: Characteristics of Ames Test Data Used In This Prediction Model
Example 1: Ames Test
Includes more than 6,000 cases of high-quality test data, which were acquired from GLP facilities.
Various structural data including dyes, liquid crystals, dispersion materials, etc., in addition to pharmaceuticals
⇒Compared to public data sets, the chemical space is large.
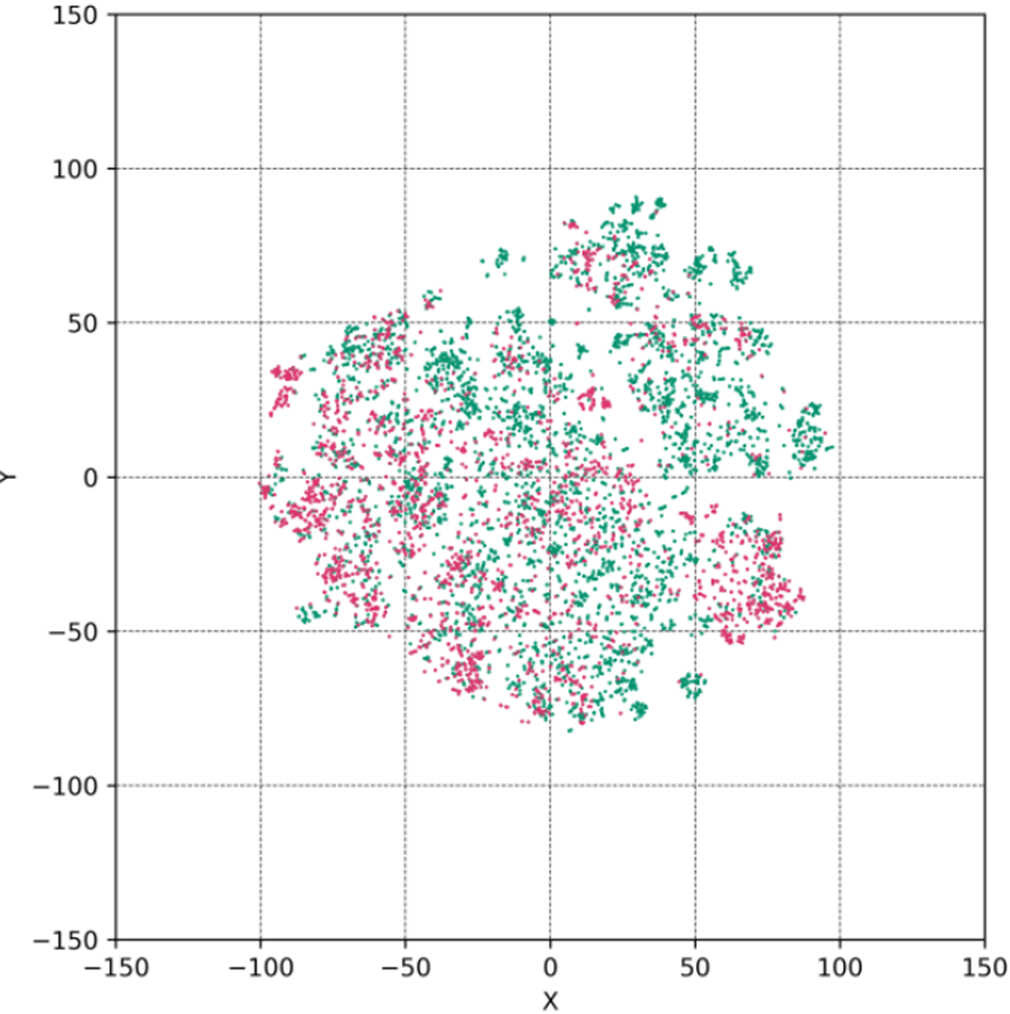
- Calculation of chemical fingerprints and compare distributions using t-SNE (nonlinear dimensionality reduction algorithm)
- This training data includes public chemical substance data.
● Public chemical substance data (NTP, J-CHECK, workplace safety sites, etc.)
● Training data of FUJIFILM model
Example 2: Skin Sensitization Test
Photographic materials contain skin sensitizers, and we possess an extensive amount of internal data on this aspect.
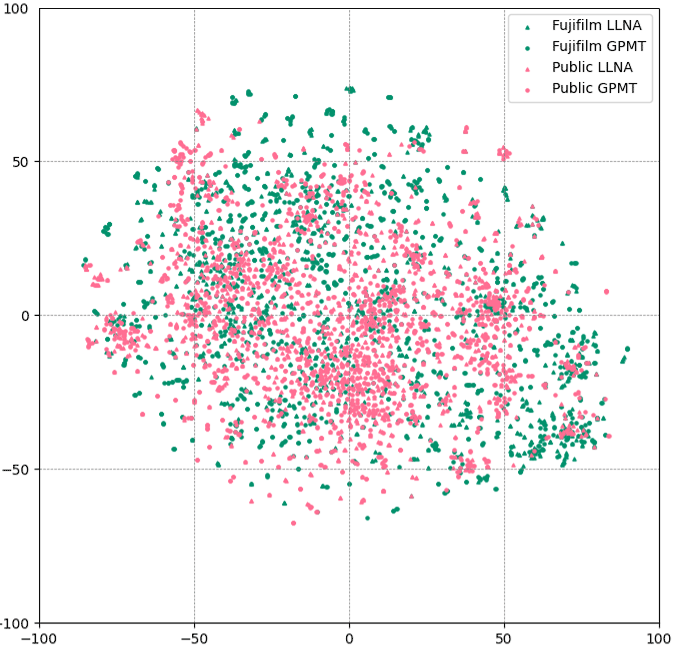
| LLNA | GPMT | |
|---|---|---|
| Public chemical substance data | 1198 | 1532 |
| Fujifilm Internal Data | 775 | 1423 |
| Total | 1953 | 2955 |
● Public chemical substance data
● Fujifilm Internal Data
Skin Sensitization Model
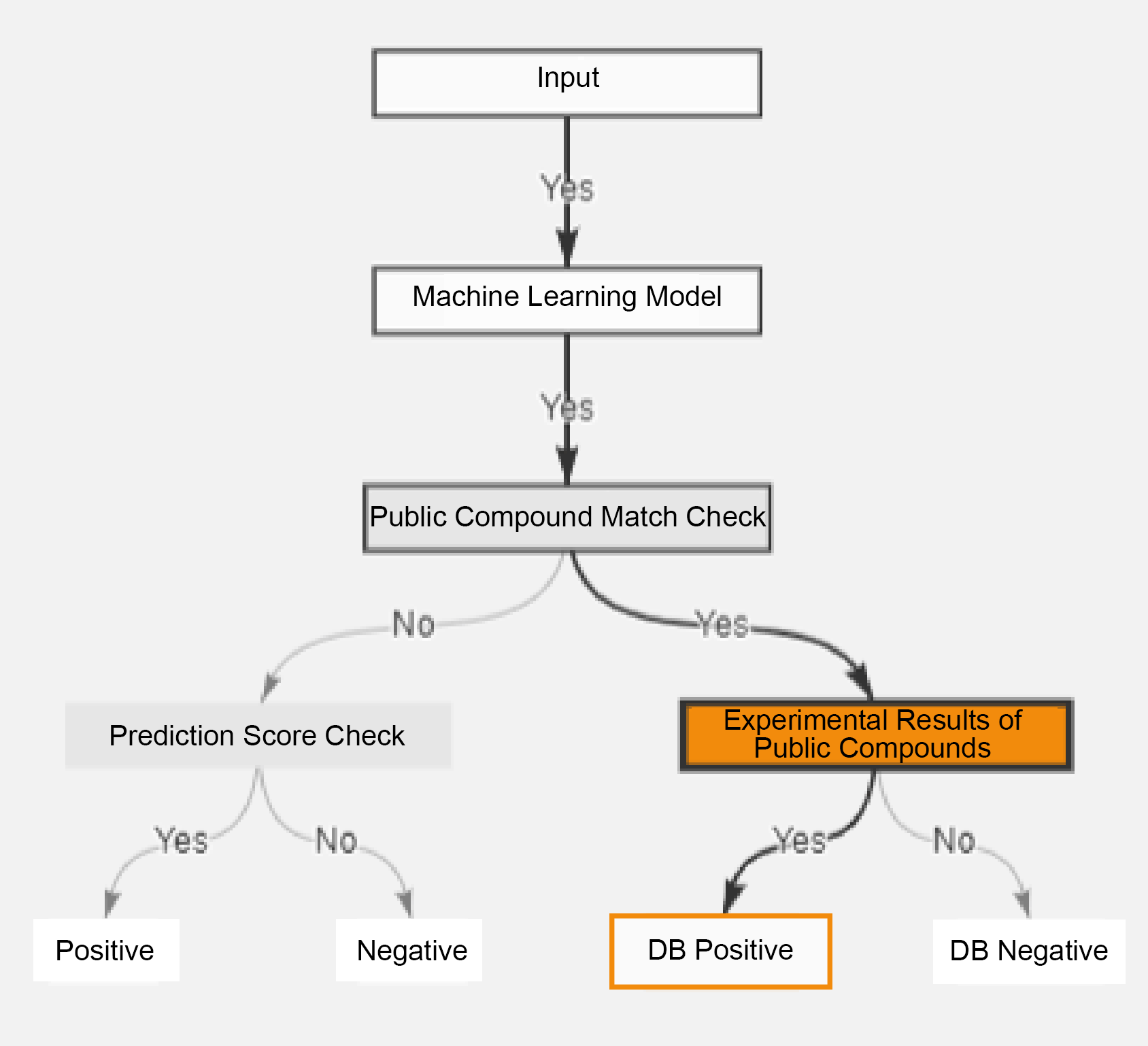
Public Database Matching Function
Poster
| Date of Conference | Presented At | Poster Title | |
|---|---|---|---|
 |
July 2, 2025 – July 4, 2025 | 52nd Annual Meeting of the Japanese Society of Toxicology | Skin sensitization in silico consensus models based on multiple Key Events using rule-based models, LLNA and GPMT statistics-based models. |
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



