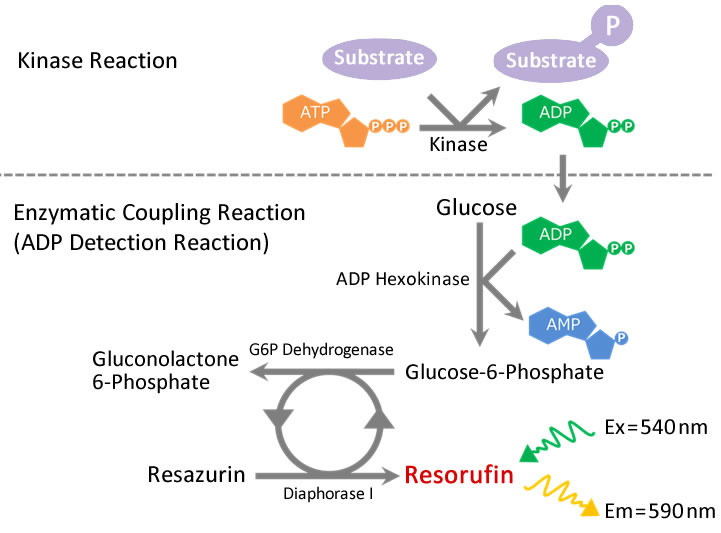
Fluorospark™ Kinase/ADP Multi-Assay Kit
Fluorospark™ Kinase/ADP Multi-Assay Kit, which is jointly-developed with Drug Discovery Initiative, The University of Tokyo, is a simple and highly sensitive ADP quantitation kit for high throughput screening (HTS) with lower cost. This kit is also applicable to ATPase, Acetyl-CoA carboxylase kinase assays as well as kinase assay which generate ADP.
Features
- Applicable to Endpoint & Real-time assay, respectively
- Excellent Z'-factor at low substrate conversion rate
- High Sensitive
- Standard curve keeps the linearity up to 30 μmol/L of ADP.
- 30 minute reaction with one-step protocol
- Lower cost
References
- Kumagai, K.et al.: Anal. Biochem., 447, 146-155 (2014).
- Drug Discovery Initiative, The University of Tokyo
Comparison with conventional (luminescent) method
| Fluorospark™ Kinase/ADP Multi-assay Kit | Conventional luminescent method (Company A) |
|
|---|---|---|
| Principle | ADP determination (Fluorescent) (Generated ADP is directly determined) |
ADP determination (Luminescent) (Converted from generated ADP to ATP and determined the ATP) |
| Pipetting step | 1 step | 2 steps |
| Operation time | 30 minutes | 70 ~ 100 minute |
| ADP measuring range | ~ 30 μmol/L | ~ 1 mmol/L |
| Price | Lower | Higher |
| Advantage | High Z'-factor Applicable to Real-time-Assay |
Wide dynamic range Higher S/B ratio |
Summary
Fluorospark™ Kinase/ADP Multi-Assay Kit is a simple and highly sensitive ADP quantitation kit through by enzymatic coupling reaction. The generated ADP through kinase reaction has been quantified as red fluorescent Resorufin. By the quantification of Resorufin, this kit can easily quantify the kinase activity with high sensitivity.
Kit Contents
| (No.) Reagent | Intended purpose | 1,000 tests | 10,000 tests |
|---|---|---|---|
| (1) Substrate Solution |
Preparation of 2 x Detection Solution
|
9 mL
|
90 mL
|
| (2) Enzyme Solution |
500 μL
|
5 mL
|
|
| (3) Resazurin Solution |
100 μL
|
1 mL
|
|
| (4) Reductant Blocker |
400 μL
|
4 mL
|
|
| (5) Stop Solution |
for stopping of the enzymatic coupling reaction
|
10 mL
|
100 mL
|
| (6) 10 mmol/L ATP Solution |
for kinase reaction and preparing a standard curve
|
100 μL
|
1 mL
|
| (7) 10 mmol/L ADP Solution |
for preparing a standard curve
|
100 μL
|
1 mL
|
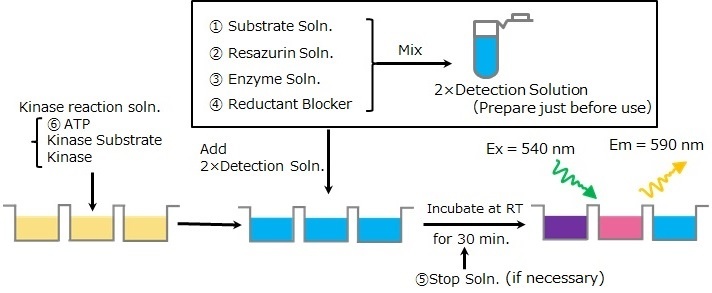
Protocol of Endpoint assay
Kinase activity can be measured by preparing and adding 2 x Detection Solution.
Performance
Preparation of Standard Curve of ADP
Prepare the standard curve using 0, 10, 20 and 30 μmol/L ADP.
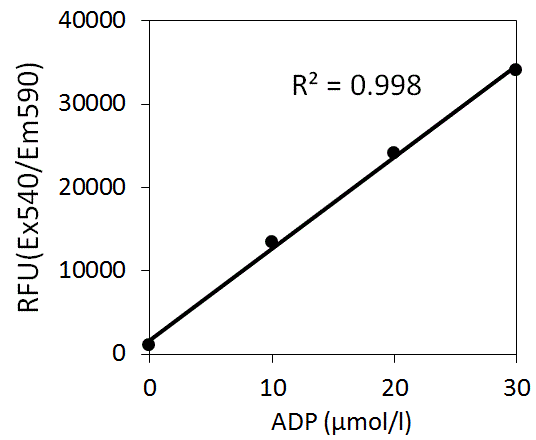
The result indicated that the standard curve keeps linearity up to 30 μmol/L of ADP.
ADP Standard Curve at the low substrate conversion rate
Prepare the several standard curve with the substrate conversion rate is 0 ~ 10% at ATP and ATP concentration is 1, 10 and 100 μmol/L
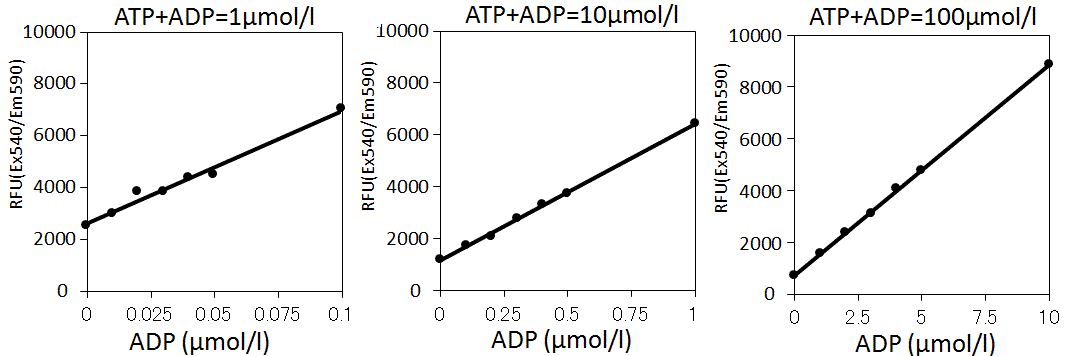
The result indicated that the quantitative capability is successful in the condition of the low substrate conversion rate (⇒ the low concentration of ADP).
Fluorescent signal stability
20 μmol/L ADP and 2 x Detection solution were mixed and after 30 minutes to 7 hours the fluorescent intensity was measured. After 30 minutes, the fluorescence intensity was standardized at 100% and the variability plotted on a graph.
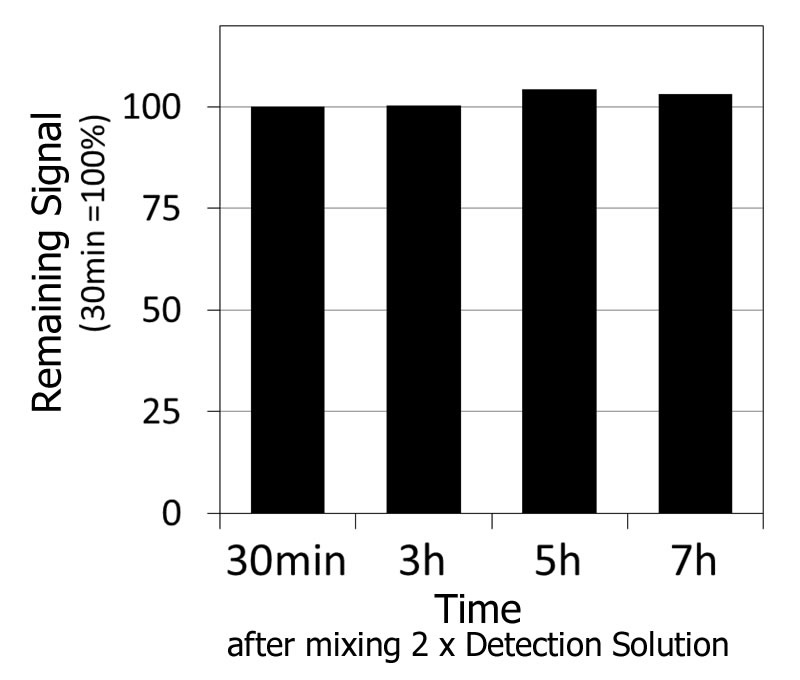
The fluorescence intensity was stabilized during the 30 minute to 7 hour interval after ADP and 2 x Detection Solution were mixed.
Data
Calculation of Z'-factor
Z' -factor was calculated at 5% Conversion Rate (ADP/ATP = 1, 10 and 100 μmol/L each)(ATP+ADP= 1, 10, 100μmol/L). The corresponding 5% substrate conversion rate of ADP was quantified and the Z'-factors of this technique and conventional spectroscopic methods were compared.
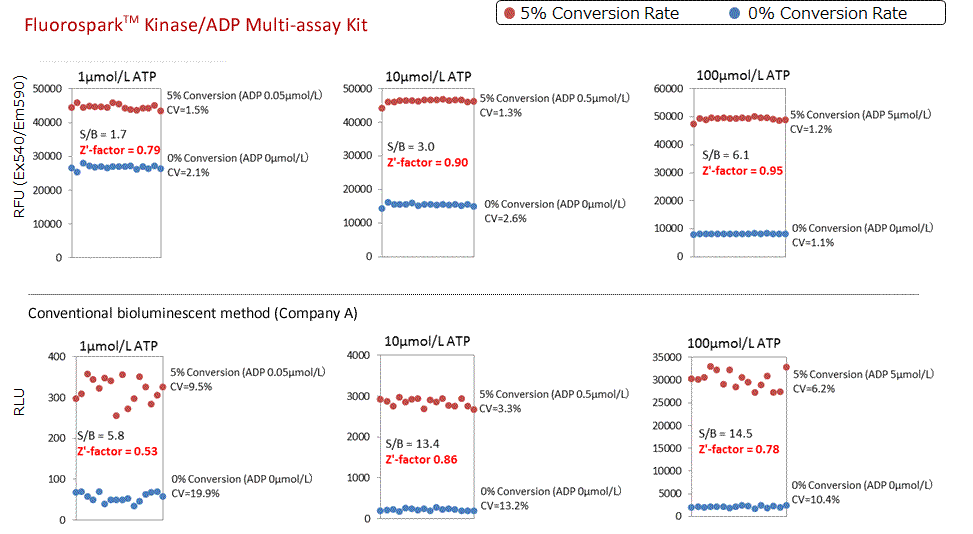
Z'-factor : Fluorospark™ Kinase > Conventional bioluminescent method (Company A). Superior Z'-factor values can be acquired even for low-substrate conversion rates of 5% with regards to the various concentrations of ATP.
Z'-factor is ...
Z'-factor is one of the static values to evaluate the performance of the HTS assay in terms of sensitivity and accuracy.
The Z’-factor > 0.5 indicates the robust assay.
Z’-factor = 1 - (3 × SDH + 3 × SDL)/(AvH - AvL)
⇒ SDH, SDL: Standard deviation of high and low control group, respectively
⇒ AvH, AvL: Mean value of high and low control group, respectively
The nearer 1 the Z’-factor is, the more sensitive and lower variable assay system this kit is.
Inhibition Curve of PKA
Preparation of the inhibition curve of cAMP-dependent protein kinase(PKA) by PKA specific inhibitor, H-89.
(PKA Catalytic Subunit 0.1 U, ATP 5 μmol/L, Kemptide 125 μg/mL)
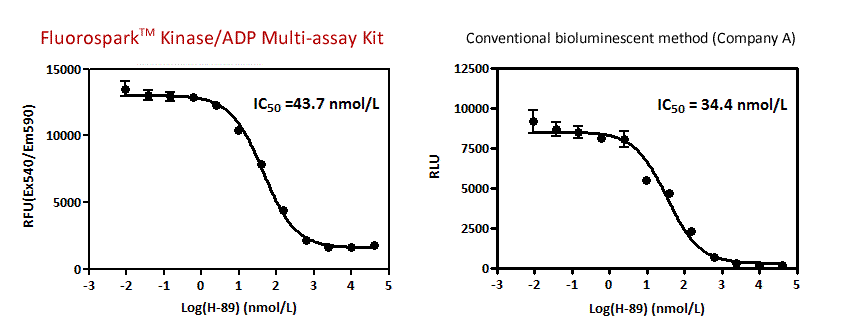
IC50 with Fluorospark™ Kinase/ADP Multi-Assay Kit is nearly equivalent to that with a conventional method (Company A).
The result indicated that the determined IC50 value was consistent with the reference's value* (IC50 = 40 nmol/L)
* Hidaka, H. et al., Methods Enzymol., 201, 328-39 (1991).
Real-time Assay Protocol
Kinase activity can be determined by measuring the fluorescence continuously.
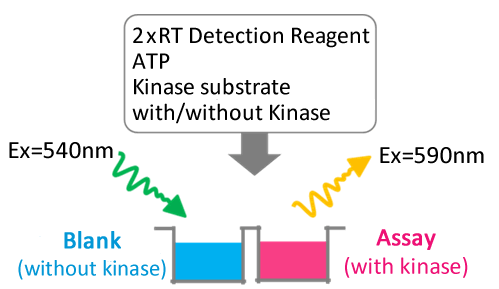
Performance
Comparison of ADP Real-time quantification time
A real-time assay was performed with various concentrations of ADP [ADP = 0, 0.25, 0.5, 1, 2 μmol/L (ATP + ADP = 10 μmol/L)]
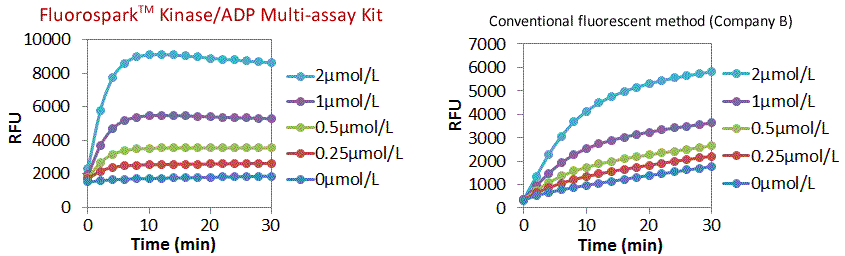
Quantitative ADP reaction were completed within approximately 10 minutes with the Fluorospark™ Kinase assay with a stable background signal (ADP = 0 μmol/L).
Influence of the addition of DTT in the Real-time Assay
A Real-time assay was performed with the addition of the reducing agent DTT (0.1, 1 mmol/L) with ADP [2 μmol/L (ATP + ADP = 10 μmol/L).
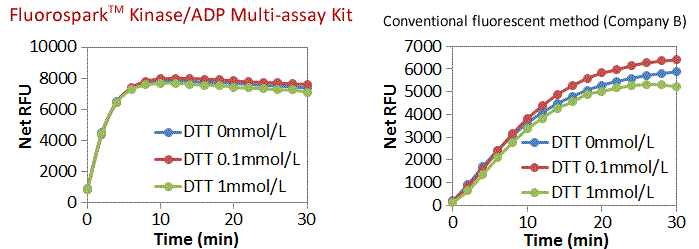
FQuantitative ADP reactions were complete within approximately 10 minutes with the Fluorospark™ Kinase assay.
Stable results were acquired even in the presence of high concentrations of DTT.
Data: Calculation of Km (ATP) of PKA by the real-time assay
(Add 50 μL of kinase reaction solution (including PKA 0.02 U/μL), the several concentration of ATP and Kempeptide 100 μg/mL)
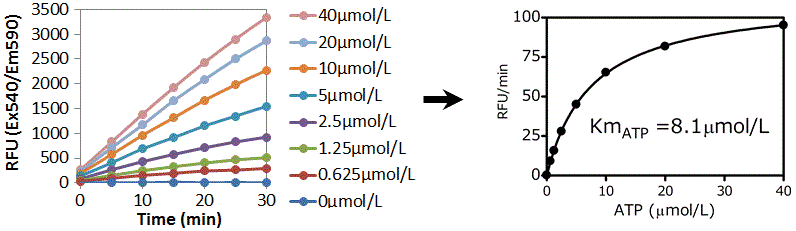
Using this real-time assay, enzyme-based kinetic analyses are feasible.
Km values obtained using this technique were approximately equal to those found in the literature (3-15 μmol/L).
** Flockhart, D.A. and Corbin, J.D.,CRC Crit. Rev. Biochem.,12, 133-86(1982).
Product List
- Open All
- Close All
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



