Nitrosamine Single Analytical Standards
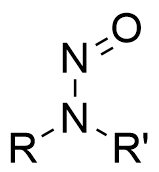
Nitrosamines are a group of compounds with a structure in which a hydrogen bound to the amine nitrogen is replaced by a nitroso group, and some of these compounds are known to be carcinogenic.
Nitrosamines are also formed as a reaction product of secondary amines and nitrous acid, so they can be detected as impurities in the manufacturing process of pharmaceuticals and are controlled under ICH M7 (Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk).
In recent years, there have been cases of nitrosamines being detected in sartan and latinidine drugs, resulting in recalls. In response, in September 2019, the EMA (European Medicines Agency) requested the marketing authorization holders to evaluate the risk of the presence of nitrosamine impurities in their drugs and take appropriate risk mitigation measures.
Recently, there is also concern about the detection of nitrosamines for which there is no carcinogenicity data, as well as nitrosamine drug substance related impurities (NDSRI). In response, on July 7, 2023, the EMA's guideline for nitrosamines EMA/409815/2020 was revised and a new evaluation method, the Carcinogenic Potency Categorization Approach (CPCA). The CPCA is a method to calculate the allowable intake of nitrosamines by classifying them into five potency categories based on the number of hydrogen atoms bound to the α-carbon of the N-nitroso group and their structural features, assuming that the carcinogenicity of nitrosamines is caused by the carbon at the α-position of the nitroso group.
We offer a wide range of analytical standards and mixture standard solutions for nitrosamines of various structures*1. This page introduces our analytical standards categorized by structure as illustrated in the CPCA's α-Hydrogen Score and Deactivating Feature Score*2.
*1 This product is not JP / Ph. Eur. / USP reference standard.
*2 As of July 2024
Analytical Standards
| Structural Classification | Structural Formula | Name | Label | CAS | Abbreviation | Code No. | Volume |
|---|---|---|---|---|---|---|---|
 |
 |
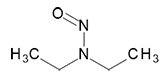
N-Nitrosomethylethylamine | - | 10595-95-6 | NMEA | 140-10001 | 50 mg |
 |
N-Methyl-N-nitrosophenethylamine | - | 13256-11-6 | NMPEA | 138-19381 | 50 mg | |
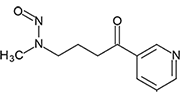 |
4-(Methylnitrosoamino)-1-(3-pyridinyl)-1-butanone | - | 64091-91-4 | NNK | 133-19451 | 100 mg | |
 |
 |
N-Nitrosodiethylamine | - | 55-18-5 | NDEA | 141-09921 | 100 mg |
| d10 | 1219794-54-3 | NDEA-d10 | 147-10011 | 50 mg | |||
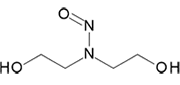 |
N-Nitrosodiethanolamine | - | 1116-54-7 | NDELA | 140-10121 | 100 mg | |
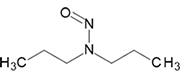 |
N-Nitrosodi-n-propylamine | - | 621-64-7 | NDPA | 140-09991 | 100 mg | |
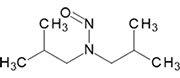 |
N-Nitrosodiisobutylamine | - | 997-95-5 | NDIBA | 143-10231 | 100 mg | |
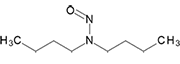 |
N-Nitrosodi-n-butylamine | - | 924-16-3 | NDBA | 149-09961 | 100 mg | |
 |
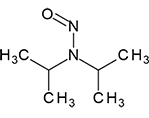 |
N-Nitrosodiisopropylamine | - | 601-77-4 | NDIPA | 145-09941 | 50 mg |
 |
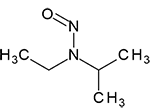 |
N-Nitrosoethylisopropylamine | - | 16339-04-1 | NEIPA | 142-09951 | 50 mg |
 |
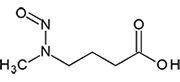 |
N-Nitrosomethylaminobutyric Acid | - | 61445-55-4 | NMBA | 146-09971 | 50 mg |
 |
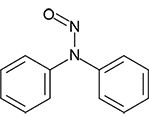 |
N-Nitrosodiphenylamine | - | 86-30-6 | NDPh | 146-10101 | 100 mg |
 |
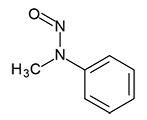 |
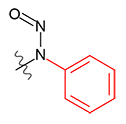
N-Nitrosomethylphenylamine | - | 614-00-6 | NMPA | 148-09931 | 50 mg |
 |
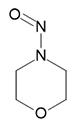 |
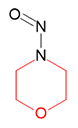
N-Nitrosomorpholine | - | 59-89-2 | NMOR | 141-10031 | 100 mg |
 |
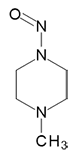 |
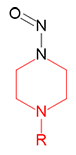
N-Nitroso-N '-methylpiperazine | - | 16339-07-4 | MNP | 143-09981 | 50 mg |
 |
 |
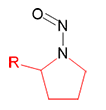
N-Nitrosopyrrolidine | - | 930-55-2 | NPYR | 143-10111 | 100 mg |
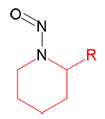 |
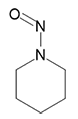 |
N-Nitrosopiperidine | - | 100-75-4 | NPIP | 149-10071 | 50 mg |
Product List
- Open All
- Close All
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.
The prices are list prices in Japan.Please contact your local distributor for your retail price in your region.



