PA tag
The PA tag is an affinity tag system of Japanese origin involving a peptide tag (GVAMPGAEDDVV) derived from PLAG sequence in human podoplanin. Anti-PA tag antibody (NZ-1) with a high affinity and specificity to the PA tag is capable of one-step purification of PA-tagged proteins to a high purity. It is also applicable to Western blotting, flow cytometry, and immunocytochemical staining.
In addition, this anti-PA tag antibody is capable of recognizing the loops of PA tagf and therefore applicable to purification/detection of membrane proteins.
Basic Information
| Tag Sequence | GVAMPGAEDDVV |
|---|---|
| Origin | Human podoplanin PLAG sequence |
| Total Number of Amino-acid Residues (aa) | 12 |
| Molecular Weight (kDa) | 1.16 |
| Isoelectric Point (pI) | 3.49 |
| Antibody Binding Affinity (KD(M)) | 4.9 x 10-10 |
| Antibody | Monoclonal antibody |
| Host | Rat |
| Clone No. | NZ-1 |
Features
High affinity and specificity of PA tag and anti-PA tag antibody
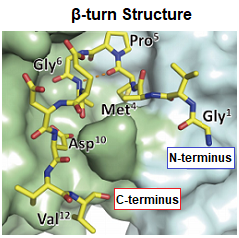
The binding between the PA tag and anti-PA tag antibody is shown to be 10-100 times stronger than antigen-antibody interactions involving other affinity tags. Reasons for this observation include the following: (1) little change in PA tag conformation upon its binding to the anti-PA tag antibody; and (2) tight fitting of the PA tag with β-turn structure into the antigen recognition site of anti-PA tag antibody.
Experiments comparing the affinity (binding strength) between tag-antibody pairs have shown that the PA tag and anti-PA tag antibody scarcely dissociate. The smaller dissociation constant (KD), an index of binding affinity between a tag and an antibody against it, indicates the stronger tag-antibody binding. The value for PA tag, 4.9 x 10-10M, shows an affinity substantially greater than those for other affinity tags.
The stronger tag-antibody binding ensures the firmer capture of the tagged target protein with the anti-tag antibody and allows application under the harsher washing conditions. Despite such a strong binding affinity, compatibility with competitive elution by addition of the tag peptide is an interesting feature of PA tag system.
Compatible with regeneration under neutral conditions
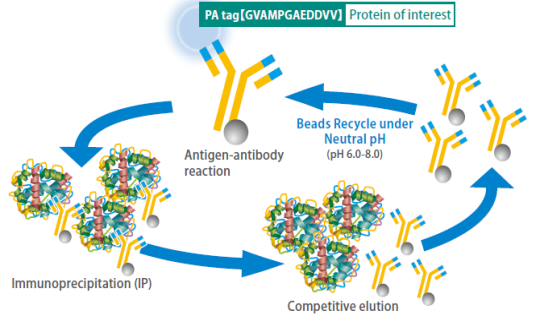
Acidic buffers (pH 2.5-3.5), usually used for regeneration of antibody-conjugated beads, damage antibody molecules and thereby reduce the efficiency of protein purification after repeated regeneration of the beads.
In contrast, anti-PA tag antibody-conjugated beads can be regenerated up to 60 times under neutral conditions without reducing the efficiency of protein purification, if a specially developed washing solution is used. This reduces costs of protein purification.
- The washing solution may not always remove tag peptides from the anti-PA tag antibody conjugated on beads completely. Watch for cross contamination when an identical batch of beads is used for purification of different proteins.
Capable of recognizing a loop structure
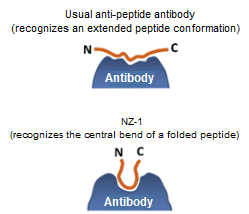
Conventional anti-tag antibodies recognize peptide tags in their extended conformation. Consequently, most peptide tags can only be added to flexible regions of a protein molecule (N- and C-termini).
However, the PA tag has a β-turn structure at the Pro-Gly bond, and the anti-PA tag antibody recognizes this β-turn structure. Taking advantage of this property, the PA tag is compatible with insertion into loops within target proteins.
The PA tag has been used for the following experiments:
- Immunoprecipitation of “integrin” with the PA tag inserted into its loop segment.
- Insertion of the PA tag into a loop segment within “semaphorin 3A” (a protein unsuitable for tagging at N- or C-termini due to adjacent functional site and active site) and successful purification of the active PA-tagged protein.
Application Data
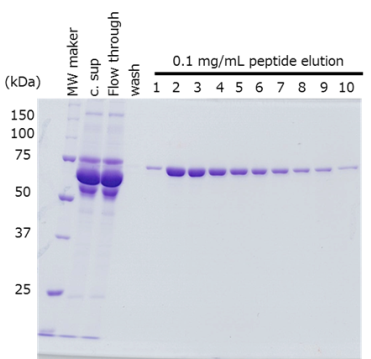
Protein purification from culture supernatant
The target protein was fused with the PA tag at the N- and C-terminus respectively, and purified from cell culture supernatant using anti-PA tag antibody-conjugated beads.
-
PA-tagged Protein at N-terminus
-
PA-tagged Protein at C-terminus
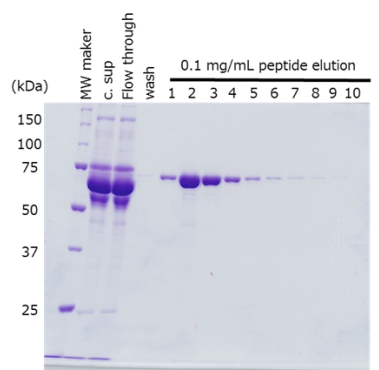
-
Sample Culture supernatant of HEK293T cells, 420 mL
(48-72 hr after transfection)Antibody anti-PA tag antibody beads (Net 4 mL) Incubation Conditions 2 hr, 2-4°C Washing Conditions Wash 5 times with TBS (20 mM Tris-HCl, 150 mM NaCl), 4 times the volume of the antibody beads Elution Conditions Add an equal volume of a PA tag peptide solution (0.1 mg/mL) to the antibody beads 10 times
The target protein was efficiently purified to a high purity, regardless of the location of PA tag insertion.
Data by courtesy of:
Dr. Junichi Takagi (Laboratory of Protein Synthesis and Expression, Institute for Protein Research, Osaka University) and Dr. Yuki Fujii (Department of Regional Innovation, Tohoku University Graduate School of Medicine)
Western blotting
Cell lysate from osteosarcoma cells expressing isocitrate dehydrogenase 1 (IDH1) fused with the DYKDDDDK tag and PA tag at the C-terminus was subjected to Western blotting.
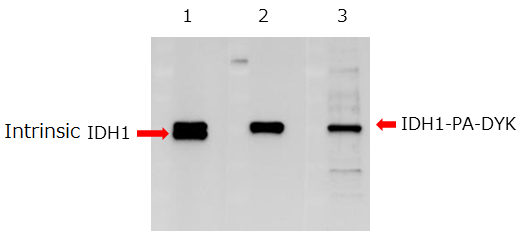 |
|
The anti-PA tag antibody recognized tagged proteins more specifically than the anti-DYKDDDDK tag antibody.
Data by courtesy of: Drs. Yukinari Kato and Mika K. Kaneko, Department of Regional Innovation, Tohoku University Graduate School of Medicine
Detection of integrin, a membrane protein
The PA tag was individually inserted into eight loop segments in integrin, a membrane protein. The tagged proteins were immunoprecipitated with the anti-PA tag antibody.
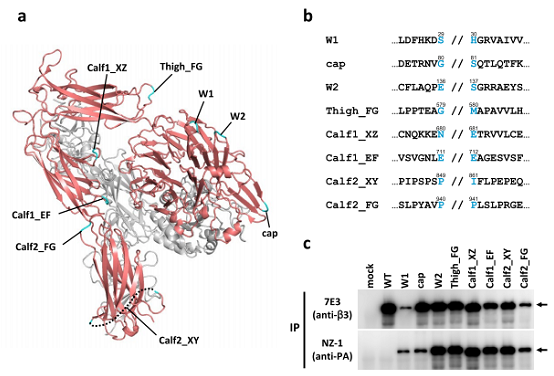
PA tag insertions into loop regions of integrin
(a) Structure of the ectodomain of integrin αIIb (red) and β3 (grey) (PDB ID: 3FCS). The loop region with the PA tag inserted is highlighted in cyan.
(b) Amino acid sequence around the insertion site. The 12-amino-acid PA tag was inserted at the position indicated by //.
(c) Immunoprecipitation of individual PA-tagged variant proteins using the anti-integrin antibody (7E3, upper) and anti-PA tag antibody (NZ-1, lower).
The inserted PA tag enabled immunoprecipitation of native integrin.
Wako Junyaku Jiho Vol. 85 No.1 (January 2017)
Authors: Drs. Junichi Takagi, Takao Arimori, and Yukiko Matsunaga, Institute for Protein Research, Osaka University
Q&A
Q. What should I consider when using the PA tag system?
A. Since the PA tag has an amino-acid sequence derived from human podoplanin, it may detect endogenous podoplanin (37 kDa) in cell strains expressing podoplanin (e.g., HEK293, COS-1, COS-7).
| Protein Purification | Since podoplanin is a membrane protein, use of the PA tag will cause no problem if the target protein is secreted extracellularly. In addition, the anti-PA tag antibody is reported not to react with endogenous podoplanin in cell lysate. |
| Western Blotting | A band of endogenous podoplanin may be detected at the position corresponding to a molecular weight of 37 kDa. |
| Immunocytochemical Staining | Use cell strains not expressing podoplanin. |
Q. Will the anti-PA tag antibody react with podoplanin from non-human animal species?
A. The anti-PA tag antibody will react with podoplanin from primates. It will not cross-react with podoplanin from mice, rats, hamsters, and dogs. Therefore, it will be applicable to protein expression in CHO cells without any problem.
Q. Give some examples of cell strains to which the PA tag system has been successfully applied.
A. Give some examples of cell strains to which the PA tag system has been successfully applied.
Q. What purpose has the PA tag system been successfully applied to?
A. It has been successfully applied to protein purification immunocytochemical staining, surface plasmon resonance immunoassay, Western blotting, ELISA, and others.
Q. What proteins have been successfully purified with the PA tag system to date.
A. Membrane proteins, serum proteins, secretory proteins, the ectodomain of a membrane protein, glycoproteins, transcription factors, and kinases have been successfully purified with the PA tag system. The molecular weight of these proteins range between 15 and 500 kDa.
Q. Competitive elution of my PA-tagged protein was unsuccessful.
A. Since the PA tag and anti-PA tag antibody bind very strongly, the resulting tag-antibody complex dissociates very slowly. For successful competitive elution, incubate for at least 5 min after addition of the tag peptide solution.
Q. Will there be any method for effective removal of contaminants in purification of a PA-tagged protein?
A. Inclusion of a pre-washing step with TBS containing concentrated NaCl (0.15-1.0 M) will be capable of removing contaminants from the final preparation of PA-tagged protein.
Product List
- Open All
- Close All
PA tag-inserted Vector
Anti-PA tag, Rat Monoclonal Antibody (NZ-1)
Labeled Anti-PA tag, Rat Monoclonal Antibody (NZ-1)
Anti-PA tag Antibody Beads (Agarose Beads)
Anti-PA tag Antibody Beads (Magnetic Beads)
PA tag Peptide (for Elution)
PA tag Peptide (for Control)
PA tag Aashing Solution
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.
The prices are list prices in Japan.Please contact your local distributor for your retail price in your region.



