CTGF(Full+N-terminal region) ELISA Kit Wako
- for Immunochemistry
- Manufacturer :
- FUJIFILM Wako Pure Chemical Corporation
- Storage Condition :
- Keep at 2-10 degrees C.
- Structural Formula
- Label
- Packing
|
Comparison
|
Product Number
|
Package Size
|
Price
|
Inventory
|
|
|---|---|---|---|---|---|
|
|
|
96Tests
|
|
In stock in Japan |
Document
Kit component
96 tests
| Antibody-coated Plate | 1 plate |
|---|---|
| CTGF Standard | 1 vial |
| Buffer | 20 mL |
| Biotin-conjugated Antibody Solution | 100 μL |
| Peroxidase-conjugated Streptavidin Solution | 100 μL |
| TMB Solution | 12 mL |
| Stop Solution | 12 mL |
| Wash Solution (10×) | 100 mL |
| Standard Sample Buffer | 100 mL |
| Plate Seal | 4 sheets |
Product Information
Connective Tissue Growth Factor (CTGF) is a secreted protein of approximately 38 kDa produced by umbilical vein and vascular endothelial cells. It is involved in cell adhesion and the proliferation and differentiation of chondrocytes. It is also considered a key factor in tissue fibrosis and reported as a potential marker of fibrosis.
CTGF contains four domains, modules 1-4. The N-terminal region (modules 1 and 2) is known as a candidate biomarker of idiopathic pulmonary fibrosis (IPF)1). However, it has been difficult to accurately measure only the N-terminal region of CTGF, because the N-terminal region and platelet-derived full-length CTGF are both present in the blood.
CTGF (Full + N-terminal region) ELISA Kit Wako is an ELISA kit for detection of full-length CTGF and N-terminal region of CTGF using monoclonal antibodies that recognize CTGF Module 1 and Module 2. The amount of N-terminal CTGF in blood can be measured when this product is used in combination with a kit for detecting full-length CTGF (CTGF (Full) ELISA Kit Wako, Code No. 290-84701)2).
-
Kit Performance
Analyte CTGF (full-length) and CTGF (N-terminal region) Sample Human serum/plasma(EDTA)
* heparin plasma is not recommendedCalibration Curve Range 7.81 - 500 pM Sample Amount Required Human serum: 5 μL
Human plasma (EDTA): 10 μLAssay Time 2 hours and 50 minutes Detection Method Colorimetry -
Principle
Data
Spiked recovery test
| Sample | Amount spiked (pM) |
Measured value (pM) |
Amount recovered (pM) |
Recovery rate (%) |
|---|---|---|---|---|
| Human Serum (ID: No.15) |
0 | 61.5 | - | - |
| 10 | 71.4 | 9.90 | 99.0 | |
| 50 | 110 | 48.5 | 97.0 | |
| 100 | 163 | 102 | 102 | |
| 150 | 215 | 154 | 103 | |
| 300 | 370 | 309 | 103 | |
| Average | 101 | |||
| Human Plasma (EDTA) (ID: No.15) |
0 | 71.5 | - | - |
| 10 | 80.8 | 9.30 | 93.0 | |
| 50 | 121 | 49.5 | 99.0 | |
| 100 | 174 | 103 | 103 | |
| 150 | 227 | 156 | 104 | |
| 300 | 380 | 309 | 103 | |
| Average | 100 |
Dilution linearity test
-
Human Serum
-
Human Plasma
Cross-reactivity
-
Cross-reactivity with each CTGF fragments
[Result]
CTGF (Full + N-terminal region) ELISA Kit Wako specifically detected the full-length and N-terminus. -
Cross-reactivity with CCN family proteins
Cross-reactivity with CCN family proteins, including CTGF, was determined (500 pM, n=8).
CCN Family Cross-reactivity (%) CCN1 N.D. CCN2 (CTGF) 100 CCN3 N.D. CCN4 N.D. CCN5 N.D. CCN6 N.D. N.D. = Not Detected
[Result]
CTGF (Full + N-terminal region) ELISA Kit Wako has high specificity for CCN2 (CTGF) among the CCN family proteins.
Comparison of CTGF reactivity between each products
Recombinant human CTGF protein (rHuman CTGF) with a known concentration (1) Full-length, (2) N-terminal region, and (3) C-terminal region were serially diluted and measured with each products. The measurement procedure followed the instruction manual of each products.
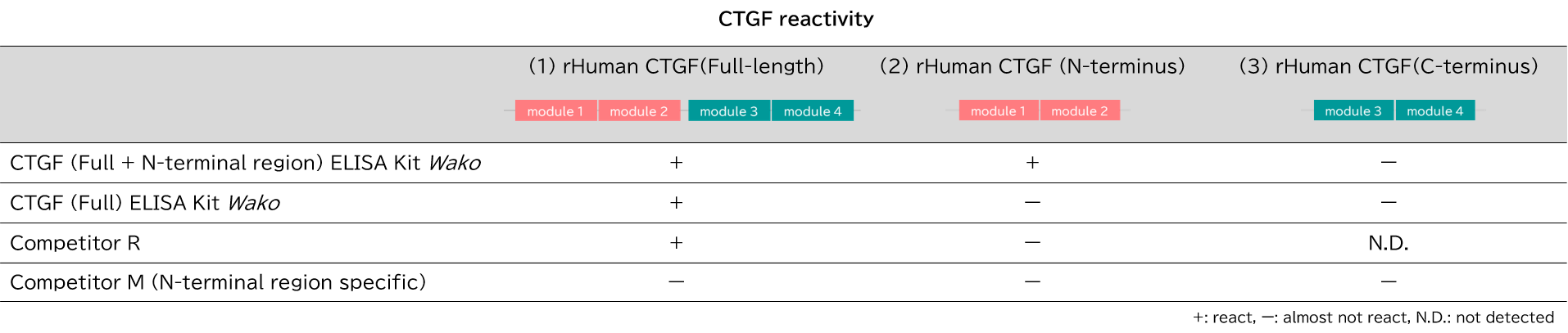
[Result]
CTGF (full length + N-terminal region) ELISA Kit Wako showed high reactivity to CTGF (full length) and CTGF (N-terminal region), and CTGF (full length) ELISA Kit Wako to CTGF (full length). The kit (competitor R) showed high reactivity only with CTGF (full length). The kit (competitor M, N-terminal region specific) hardly reacted with any of the CTGF in our examination.
Measurement of the N-terminal region (dilution linearity test)
Full-length plus N-terminal region of CTGF and the only full-length CTGF were measured using the CTGF (Full + N-terminal region) ELISA Kit Wako and the CTGF (Full) ELISA Kit Wako (Code No. 290-84701), respectively. The amount of CTGF (N-terminal region) was calculated from the difference between the two measurements, and dilution linearity was determined.
[Result]
The amount of CTGF (N-terminal region) measured using this method showed excellent dilution linearity.
Measurement of N-terminal CTGF in plasma from healthy subjects and IPF patients
The N-terminal CTGF levels were determined in EDTA plasma collected from healthy subjects and patients with idiopathic pulmonary fibrosis (IPF) using the CTGF (Full + N-terminal region) ELISA Kit Wako and CTGF (Full) ELISA Kit Wako.
[Result]
The N-terminal CTGF levels in plasma in IPF patients were significantly higher than those in healthy subjects.
References
References
- Kono, M. et al.: Clin. Chim. Acta, 412, 2211(2011).
Plasma CCN2 (connective tissue growth factor; CTGF) is a potential biomarker in idiopathic pulmonary fibrosis (IPF) - Miyazaki, O. et al.: Ann. Clin. Biochem., 47, 205(2010).
Subtraction method for determination of N-terminal connective tissue growth factor
Publications using this product
Kogiso, T. et al.: PLoS One, 19(1), e0296375(2024).
Serum level of full-length connective tissue growth factor reflects liver fibrosis stage in patients with Fontan-associated liver disease
Morishima, N. et al.: Pract. Lab. Med., 40, e00402(2024).
Serum levels of the N-terminal fragment of connective tissue growth factor is a novel biomarker for chronic pancreatitis
FAQ
About sample preparation
- What anticoagulant should be used for blood samples?
- The literature referenced below indicates that the values for N-terminal CTGF are higher when using heparin plasma than when using EDTA or citrate plasma. Heparin should be avoided as an anticoagulant.
[Reference]
Miyazaki, O. et al.: Ann. Clin. Biochem., 47, 205(2010).
About the kit
- Are the antibodies used in this kit available for purchase?
- Anti CTGF Module 1 monoclonal antibody (30D2) (Product Number: 018-27423)
The detection antibody is not sold separately. The antibody for Module 2 (Product Number: 015-27433) is available, but the clone is different from the one used for the antibody in this kit.
- How is the standard quantified?
- The standard is prepared and quantified according to the following literature.
[Reference]
Miyazaki, O. et al.: Ann. Clin. Biochem., 47, 205(2010).
About kit usage
- What reagents, instruments, and equipment are required for the assay using this kit?
- Purified water(distilled water)
- Test tubes for dilution of standard solution and samples
- Glass utensils for dilution of Wash Solution (graduated cylinder, beaker)
- Pipettes with disposable tips (one capable of pipetting 10μL of liquid accurately and one capable of pipetting 200 to 500 μL)
- Repeater pipette, capable of repeatedly dispensing 100μL
- Water-absorbent material such as a paper towel (to remove any solution remaining on the plate after washing)
- Vortex-type mixer
- Microplate shaker (range approx. 500 to 800 rpm)
- Automatic washer for 96-well plate (if available) or washing bottle
- Microplate reader capable of measuring at 450 ± 10 nm, with the correction wavelength set at 600 to 650 nm
- Software for data analysis
- Is it necessary to always use this kit with the CTGF (Full) ELISA Kit Wako (Product Number: 290-84701)?
- Both this kit and the CTGF (Full) ELISA Kit Wako are required only when measuring the N-terminal CTGF (Module 1 - 2).
- How many samples can be measured using the 96 tests?
- Since 16 wells are needed for standards (n = 2), 80 samples (n = 1) or 40 samples (n = 2) can be measured.
- Can the kit be divided for use?
- Yes, it can. The plate can be cut at every eight-well boundary, and lots are available for standard use. It should be noted that fewer samples can be analyzed when the kit is divided for use, because a calibration curve needs to be drawn each time.
Overview / Applications
Property
Manufacturer Information
Alias
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.




