Nitric Acid (1.38)
- for Boron Determination
- Manufacturer :
- FUJIFILM Wako Pure Chemical Corporation
- Storage Condition :
- Protect from light.
- CAS RN® :
- 7697-37-2
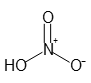
- Molecular Formula :
- HNO3
- Molecular Weight :
- 63.01
- GHS :
-
- Structural Formula
- Label
- Packing
- SDS
|
Comparison
|
Product Number
|
Package Size
|
Price
|
Inventory
|
|
|---|---|---|---|---|---|
|
|
|
500mL
|
|
In stock in Japan |
Please check here for notes on products and prices.
Document
Overview / Applications
| Outline | An aqueous solution of HNO3, which is a monobasic strong acid. A concentrated solution of HNO3 is a powerful oxidizing agent and dissolves many metals. It is used for synthesizing inorganic and organic nitrates, nitrate esters and nitro-compounds and producing ammonium nitrate. It is decomposed by light and heat to generate nitric monoxide, oxygen, etc. Contact with the skin and mucosa will result in a burn and corrosion. A solution of HNO3 at a concentration 98 to 99 %, in particular, is referred to as fuming nitric acid. A reagent with a boron (B) content maintained at equal to or less than 20 ppb. When preparing a sample solution specified in JIS G1227 "Method for determining boron in iron and steel", it is used for the hydrolysis of the sample. |
|---|
Property
| Appearance | Colorless clear liquid |
|---|---|
| Density | about 1.38g/mL |
| Concentration | 60 - 61% (Titration) |
Manufacturer Information
Alias
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.
The prices are list prices in Japan.Please contact your local distributor for your retail price in your region.